Table of Contents
ToggleCram’s rule of asymmetric induction predicts the stereochemistry of the nucleophilic addition to a carbonyl compound with an adjacent chiral center. Therefore, it is a very important guidance in organic chemistry. It was given, first of all by Cram in 1952.
Cram’s rule of asymmetric induction
When a molecule with one or more chiral centers creates a new one, two diastereomers are generated in unequal proportions. Such reaction is called Asymmetric synthesis and the existing chiral center is said to bring about asymmetric induction.
Cram’s rule organic chemistry
Cram’s rule helps to predict the stereochemistry of the nucleophilic addition to a carbonyl group with an adjacent chiral center. The best conformation for nucleophilic addition to carbonyl compounds with an adjacent chiral center (without polar groups) is considered to be one that minimizes the interaction during the entry of the nucleophilic group between the largest group and the carbonyl group reagent.
When a ketonic group attached to a chiral center having a large, a medium and a small group undergoes nucleophilic addition with organometallic or metal hydride reagents, two diastereomeric products result. Among these two diastereomeric products( erythro and threo), one predominates. In such a case, the relative configuration of the predominant isomer is predicted by Cram’s rule based on some arbitrary models.
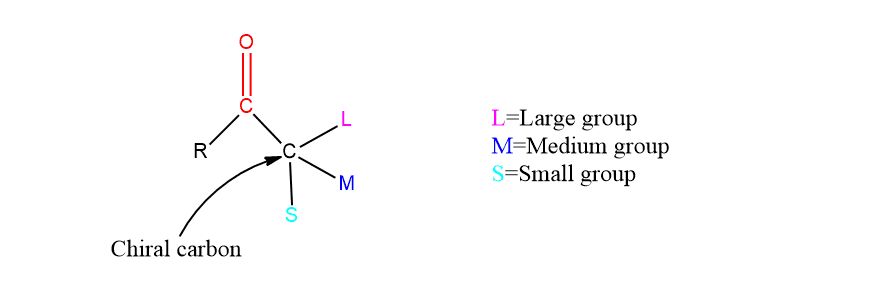
Cram’s rule states that the carbonyl group is flanked by two groups M(medium) and S(small), the incoming group preferentially attacks the side containing a small group.
Let’s see how this rule works. It will be easy to understand by observing the following models.
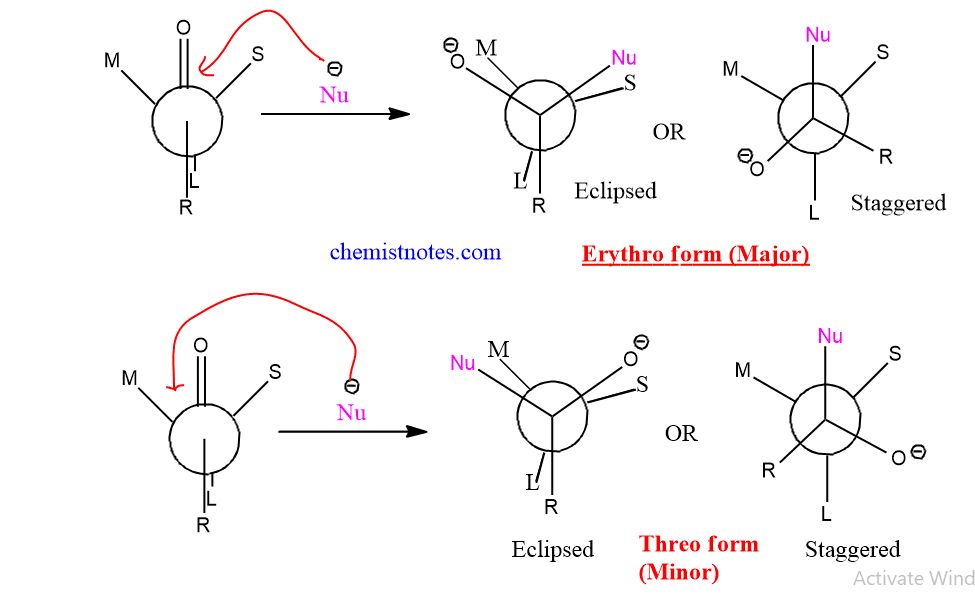
Basic guidelines of Cram’s Rule
The basic steps in Cram’s rule are explained below.
- First of all, assign the Large group(L), Medium(M) and small(S) groups at the chiral center.
- Determine the orientation of Large group with carbonyl group. If L group is syn to carbonyl group then, nucleophile will attack from the side similar to medium group.
- If L group is Anti to carbonyl group then, nucleophile will attack from the side similar to smaller group
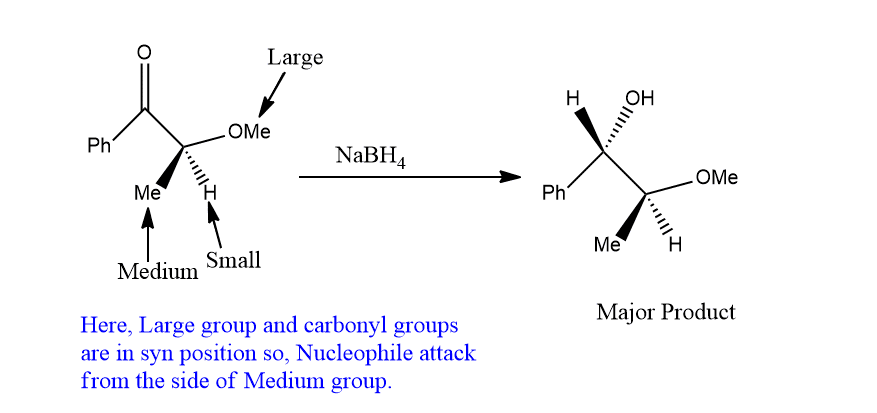
cram’s rule example
2-phenyl propionaldehyde when treated with Methyl magnesium Bromide(CH3MgBr), erythro-3-phenyl butane-2-ol is obtained as the major product. Let’s see an example.
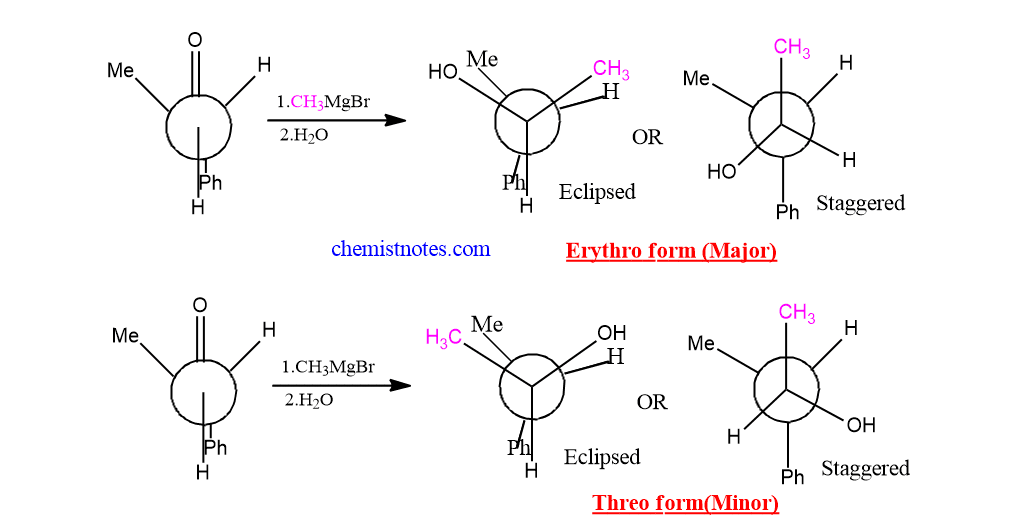
Application of Cram’s Rule
When benzoin having S OR R configuration is reduced with lithium aluminum hydride, meso-hydrobenzoin is obtained as the major product. This can be explained by using Cram’s rule.
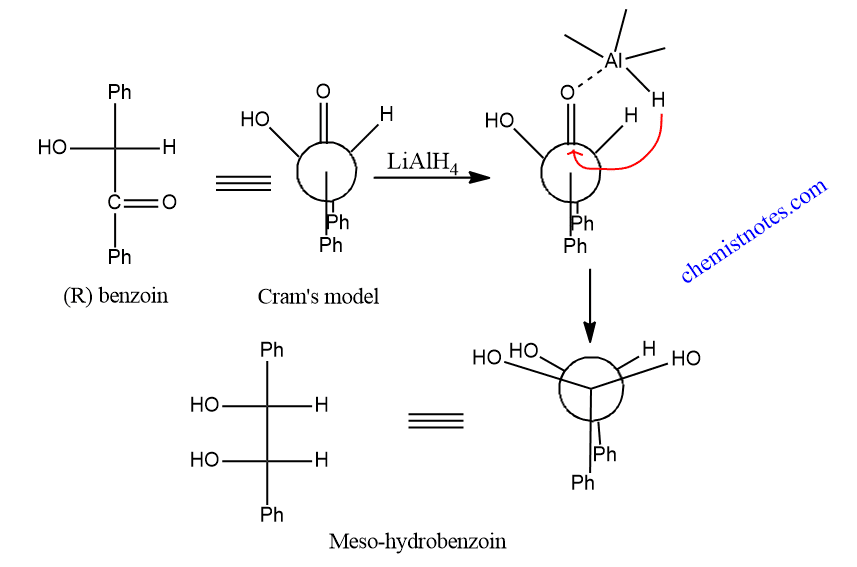
Hydrogen atom from LiAlH4 attacks the benzoin from the less hindered side to give meso-hydrobenzoin as the major product.
cram’s rule in stereochemistry
References
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010






