Table of Contents
TogglePrelog’s rule predicts the stereochemical outcome of nucleophilic addition reaction with α-ketoesters. This rule explains the empirical correlation between the arrangement of the groups in the isomer which is predominately formed and the corresponding arrangement in the optically active alcohol taken.
It is the extension of Cram’s rule that explains the formation of an unequal amount of two diastereomers in the case of chiral α-ketoester. According to this rule, the attacking group ( will move toward the side of the plane with the smaller group on the asymmetric carbon when it approaches the triangular face of the double bond in a chiral α-ketoester.
Prelog’s rule asymmetric synthesis
When a new chiral center is created in a molecule that already contains one or more, two diastereomers are formed in unequal amounts. The reaction is known as asymmetric synthesis and the existing chiral center is said to bring about asymmetric induction.
Prelog’s rule asymmetric induction
With ketoesters of the type R-CO-CO-OC*SML, where S, M, and L are small, medium, and large groups, respectively, on the chiral center, Prelog’s rule predicts the stereochemical outcome of the nucleophilic addition reaction. The S, M, and L groups of the rest of the molecule, R-CO-CO-OC*-, which lie in the plane, have three favorable conformations: a) in the plane, b) in front of the plane, and c) behind the plane.
It is important to remember that the two C=O groups must be trans and that the S, M, and L groups, which are in the same plane as the rest of the molecule, must face the same direction as the carbonyl group connected to R of group R-CO-CO-OC*SML, must be trans.
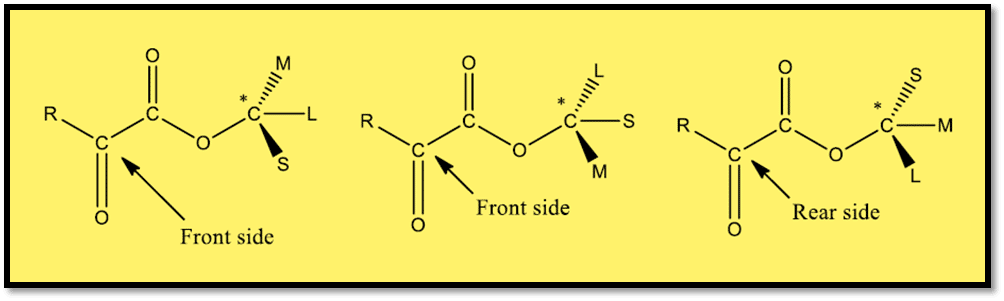
Prelog considers the following conformations for the parent ester to be equally likely, therefore they should be taken into consideration. The two faces of the carbonyl group are now diastereotopic, and the prevailing diastereoisomer would be the one in which the reagent (let’s say RMgX) approaches from the side that is sterically less hindered, which is governed by the two groups of S, L, and M that are out of the plane.
In simple words, the large group and the group associated with the keto group should be on the opposite side of each other. The incoming nucleophile in this instance chooses to attack the keto carbonyl group from the side of a small group.
Because S is the smallest between the two groups M and S, it will be preferable to apply the reagent from the front side.
Steps of Prelog’s rule
Let us consider the keto ester of the following types.
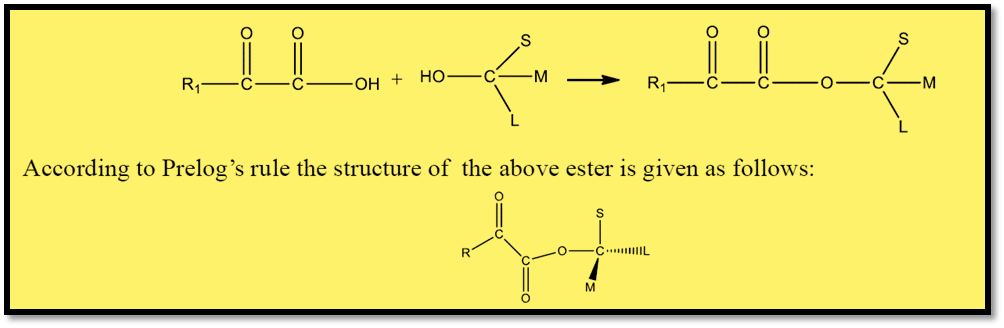
- It is necessary to specify how the groups are arranged in the ketoester, the starting material.
- The two carbonyl groups are often positioned in such a way that they are anti-parallel to one another and that the smallest group (S) in the alcohol portion is eclipsed by the ketone carbonyl.
- The reagent R’ attack the carbonyl group from the medium-sized M which is the smaller of the two groups in the alcohol part.
Prelog’s rule for the synthesis with α-keto acids as described above can be explained using (-) menthol. In this case, the small group S is H, the medium-sized group M is methylene of C2, and the large group L is methine replaced with the isopropyl group at C4.
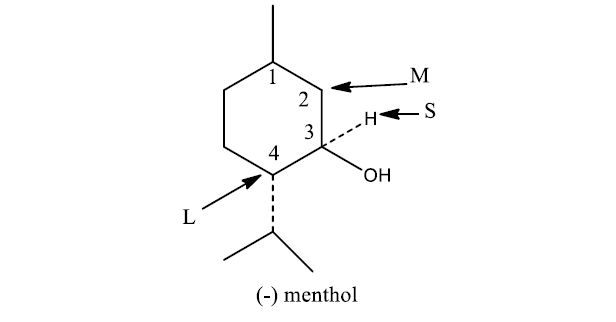
Cram’s and Prelog’s rule
Cram’s rule governs the stereochemistry of the nucleophilic addition to a carbonyl group with an adjacent chiral center. According to Cram’s rule, when a carbonyl group is flanked by two groups, M (medium) and S (small), the incoming group will approach from the side containing a smaller group if If L 9large) group is Anti to a carbonyl group. If the L group is syn to the carbonyl group then, nucleophiles will attack from the side similar to the medium group.
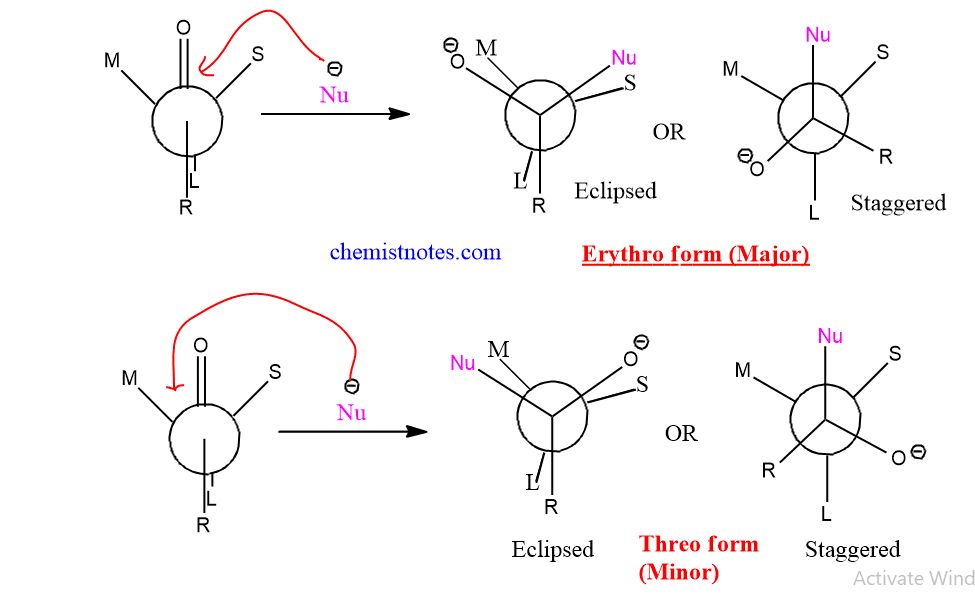
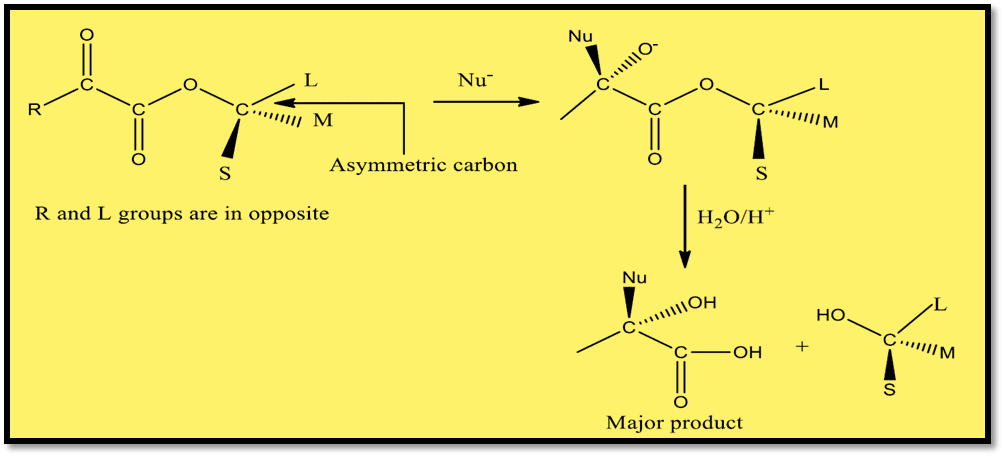
Prelog’s rule is the extension of Cram’s rule that is used to predict the stereochemistry of nucleophilic addition to α-ketoesters. The basic condition for Prelog’s rule is that the R group (attached to keto ester) and L (large) group attached to asymmetric carbon should be opposite to each other. If so, nucleophiles will approach from the small group side.
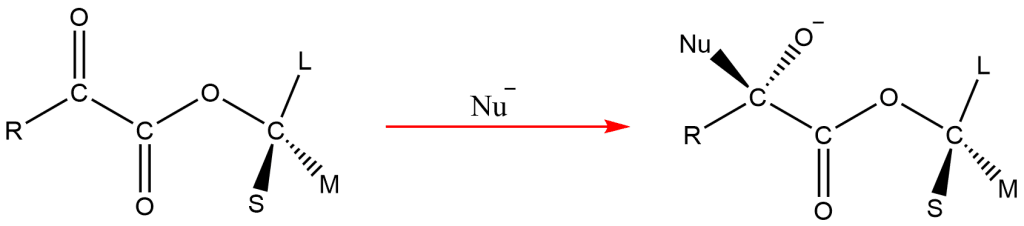
However, if L is not anti to the R group, then the asymmetric carbon is rotated to make it anti to the R group. Then the nucleophile will attack from the side of a small group. After this process, the asymmetric carbon should be rotated to make the configuration as of starting material. Further hydrolysis results the major product as illustrated below.
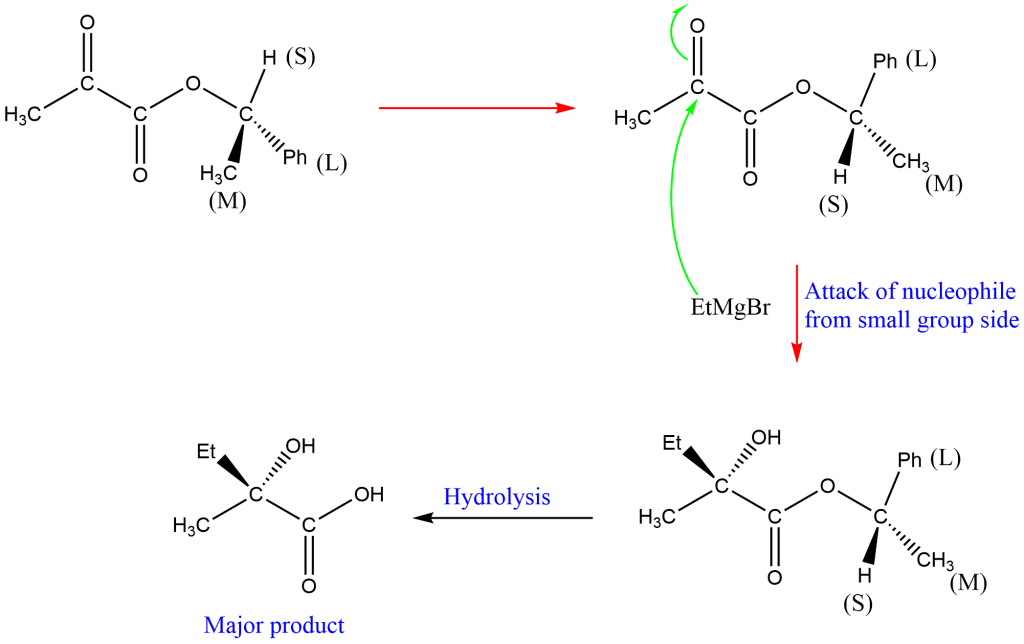
Application of Prelog’s rule
1. It helps to predict the stereochemistry of the product resulting from nucleophilic addition reaction with ketoesters.
2. Prelog’s rule is used to determine the configuration of hydroxy acid. For this, the following points should be considered.
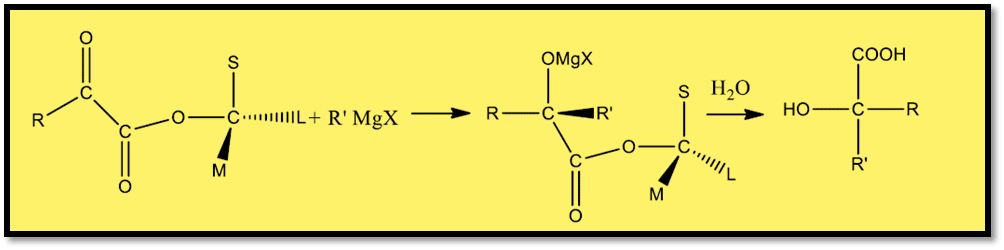
- To produce hydroxy acid, the ester of α-keto acid and optically active alcohol with a specified configuration is either reduced or subjected to react with Grignard reagent treatment.
- The predominated enantiomer’s configuration is known, under Prelog’s rule.
- The ester is quantitatively hydrolyzed to yield the known configuration of acid, after which its rotation is determined.
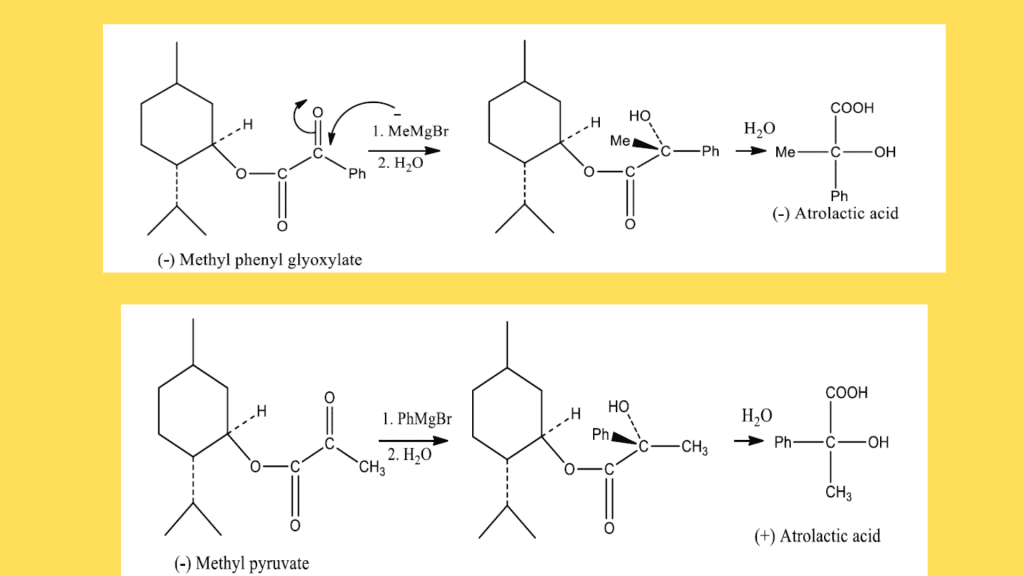
3. Similarly, the configuration of an unknown alcohol can also be determined by Prelog’s rule.
- Phenyl glyoxylic acid is used to esterify the alcohol.
- Methyl magnesium iodide is used to treat the resulting ester.
- To form the atrolactic acid, the ester is hydrolyzed.
- With the use of a polarimeter, atrolactic acid rotation is observed.
- The configuration of atrolactic acid governs the configuration of an unknown alcohol.
4. Moreover, Prelog’s rule is used to assign the absolute configuration of the steroid molecule.
Prelog’s rule video
References
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010







One Response
Thanks