Table of Contents
TogglePeterson Olefination Reaction, Mechanisms, examples, and applications in organic chemistry are discussed here.
Peterson Olefination Reaction
The reaction involving the addition of an α-silyl carbanion to a carbonyl compound (aldehyde or ketone) to form β-silyl alcohol (or β-hydroxy silane) followed by the elimination of silol yields alkenes. Such a reaction is called Peterson Olefination reaction. This reaction is also known as Peterson elimination and Peterson reaction, or sometimes Peterson alkenation or Peterson alkenylation.
It simply leads to the synthesis of alkenes from α-silylcarbanions. Peterson Olefination reaction is closely related to Wittig reaction but offers more advantages in terms of good Z-selectivity, higher reactivity of α-silyl carbanions towards carbonyl compound (aldehyde or ketone) as well as purification procedure that leads to the formation of the product without side product like phosphorus.
In general, the α-silyl alcohol produced by a β-silyl carbanion with an electron-withdrawing group undergoes immediate elimination, yielding the olefin as an E/Z mixture with a preference for E olefin, whereas the β-silyl alcohol produced by an α-silyl carbanion with an electron-donating group can be isolated and converted into the corresponding olefin with controlled stereochemistry.
Anti-elimination occurs when the isolated β-silyl alcohol is treated with acid, whereas syn elimination occurs when the identical substrate is treated with a base. Since the results of acid or base-induced elimination differ, the Peterson Olefination provides the way to increase the yield of the desired alkene stereoisomer by cautiously separating the two diastereomeric β-hydroxy silanes followed by two different eliminations.
Generally, Fluoride such as CsF and zinc bromide is used as catalysts, while acids such as sulphuric acid and trifluoroacetic acids are used as elimination reagents
Peterson Olefination Example
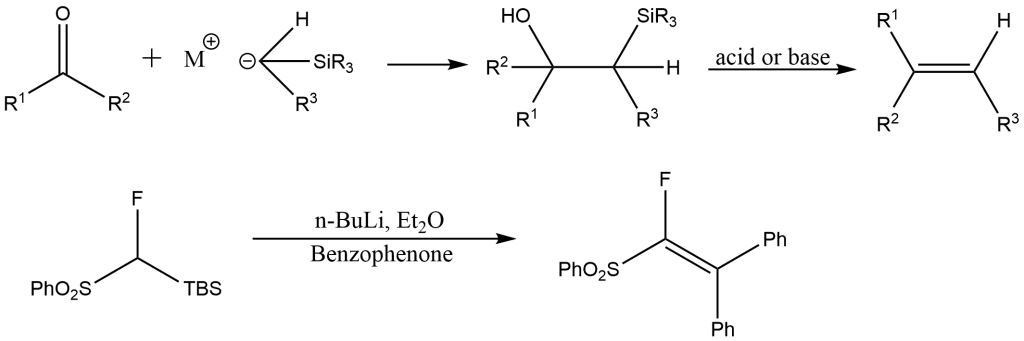
Some of the examples of Peterson olefination reactions are:
Peterson Olefination Mechanism
Peterson olefination reaction leads to the preparation of either cis- or trans-alkenes from the same β-hydroxysilane. In the reaction, the intermediate β-hydroxysilane can be separated, and the Peterson Elimination can be done afterward.
On the addition of the silyl carbanion to a carbonyl compound, β-hydroxysilane is obtained.
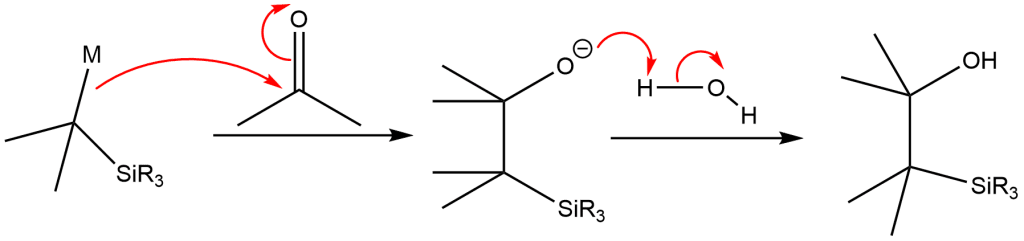
β-hydroxysilane when treated with acid and base independently, elimination occurs and leads to the formation of different isomers of alkenes.
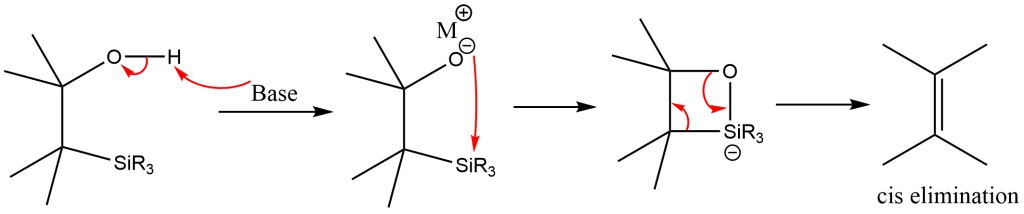
Basic elimination
syn/cis elimination occurs when β-hydroxysilane is treated with base, and hence yields desired alkenes.
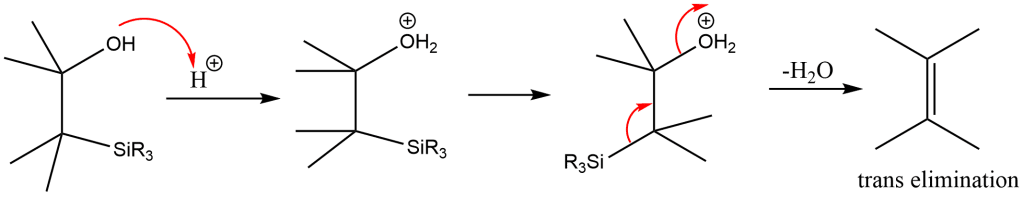
Acidic elimination
Treatment of the β-hydroxysilane results in the formation of the desired alkene via trans/anti-elimination.
Application of Peterson Olefination Reaction
This reaction has general application in the preparation of olefins.
Peterson Olefination Video
FAQs/MCQs
What is peterson olefination reaction?
Peterson olefination reaction is such an organic reaction that synthesizes desired alkene when α-silyl carbanion is added to a carbonyl compound followed by elimination.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Peterson, D. J., J. Org. Chem., 1968, 33, 780.
- Ahmad, N. M. Peterson olefination. In Name Reactions for Homologations-Part I; Li,J. J., Corey, E. J., Eds., Wiley & Sons: Hoboken, NJ, 2009, pp 521−538. (Review).
- Kano, N.; Kawashima, T. The Peterson and Related Reactions in Modern CarbonylOlefination; Takeda, T., Ed.; Wiley-VCH: Weinheim, Germany, 2004, 18−103. (Review).






