Table of Contents
ToggleMannich reaction, examples, mechanism, and application have been discussed in this post. This reaction was first of all given by Mannich and Kroesche in 1912.
Mannich reaction definition
Mannich reaction is a multicomponent condensation between an enolization carbonyl compound, a non-enolizable carbonyl compound, and an amine to form a β-amine carbonyl compound or β-Amino Ketone or β-amino ester.
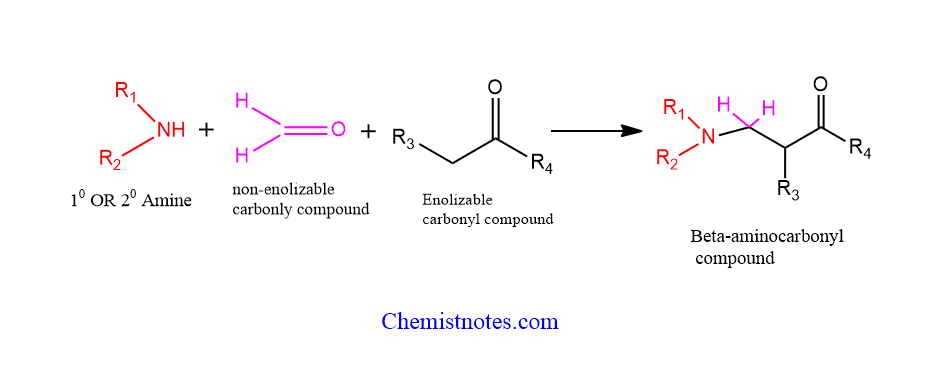
The enolizable carbonyl compound acts as a donor and the non-enolizable carbonyl compound acts as an acceptor. The amine may be primary or secondary and generally ammonia as well. Mostly, formaldehyde is used as a non-enolizable carbonyl compound. You may have a question, what are enolizable carbonyl compounds?
Those aldehyde or ketones having alpha-hydrogen are enolizable and those with no alpha-hydrogen are called non-enolizable carbonyl compounds.
- This reaction can be performed under both acidic and basic condition.
Mannich reaction examples
Let’s see an example of this reaction.
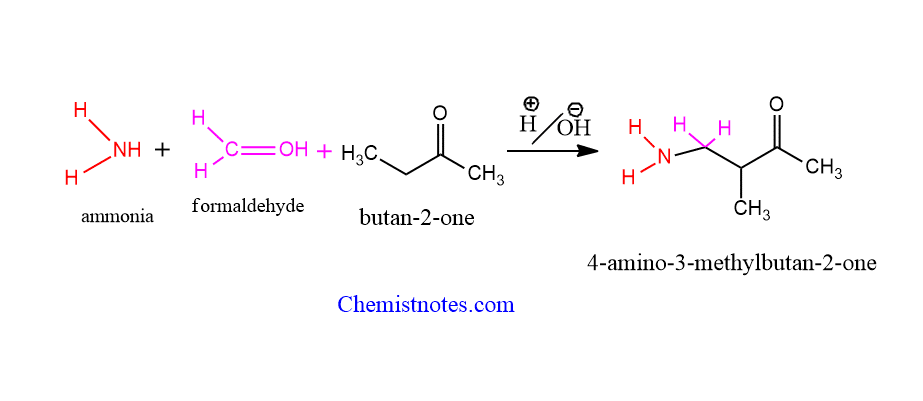
Mannich reaction mechanism
The mechanism of the mannich reaction can be explained in both acidic and basic conditions.
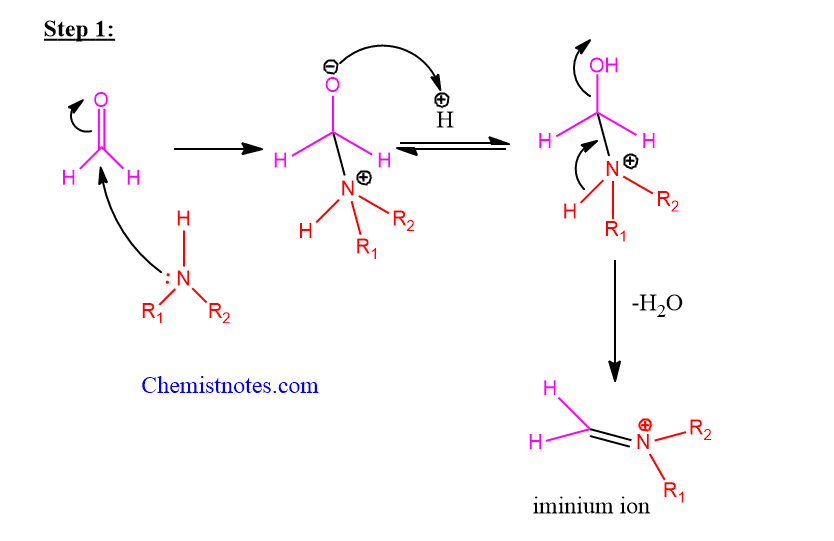
A. Mechanism Under the acidic condition
Step 1: Nucleophilic addition of amine or ammonia to non-enolizable carbonyl compounds followed by protonation to give an adduct known as iminium ion.
Step 2: Enolization of the enolizable carbonyl compound.
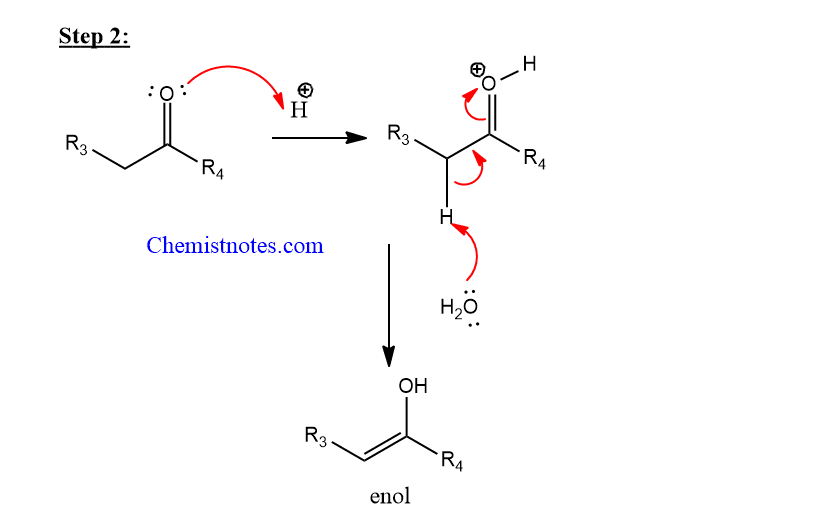
Step 3: The iminium ion reacts with enol formed in step 2 to give Mannich base.
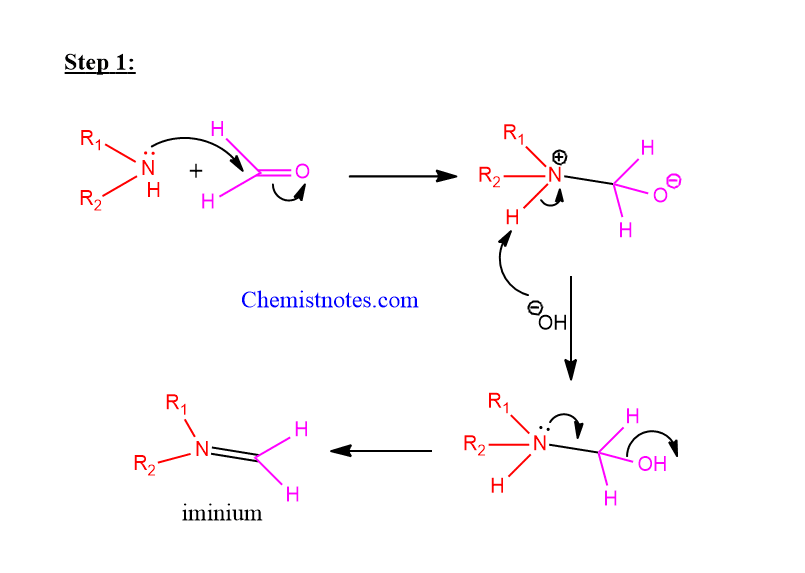
B. Mechanism under the basic condition
Step 1: Nucleophilic addition of an amine to a non-enolizable carbonyl compound.
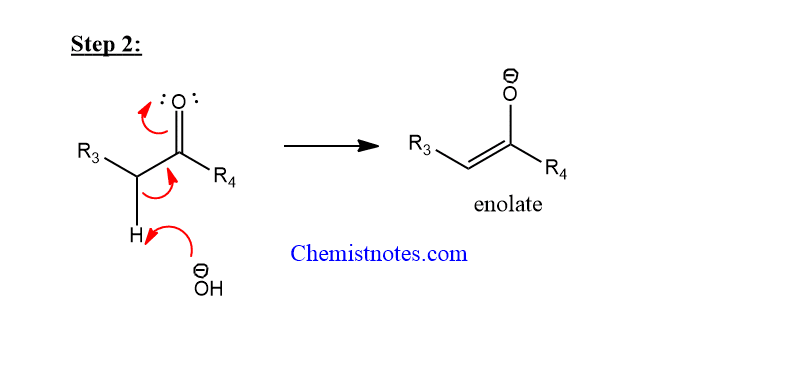
Step 2: Base abstracts hydrogen from enolizable carbonyl compound to form enolate.
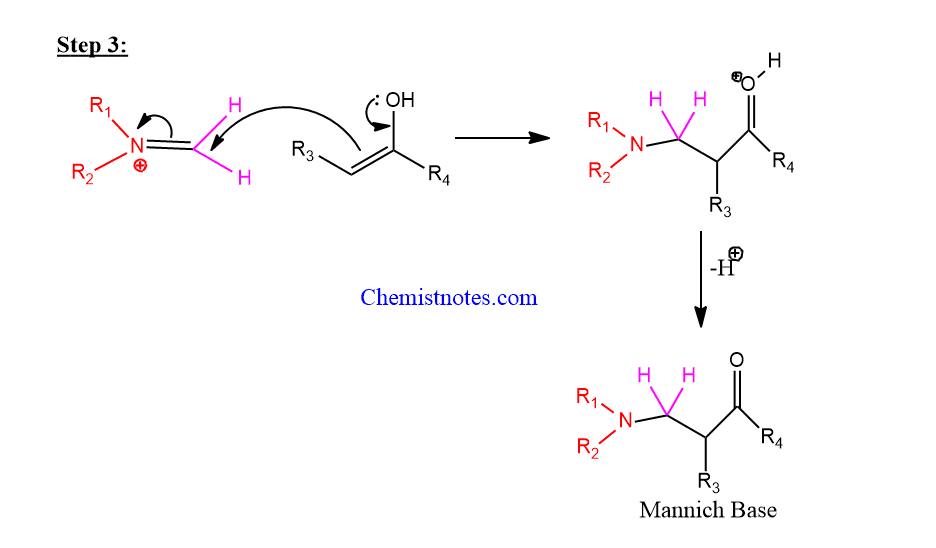
Step 3: Reaction between enolate and iminium takes place.
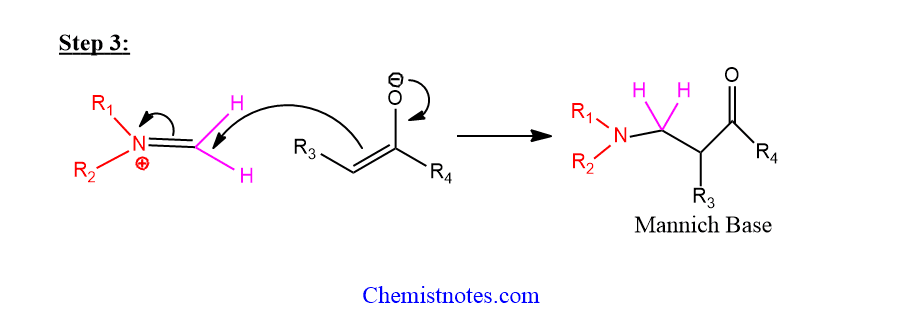
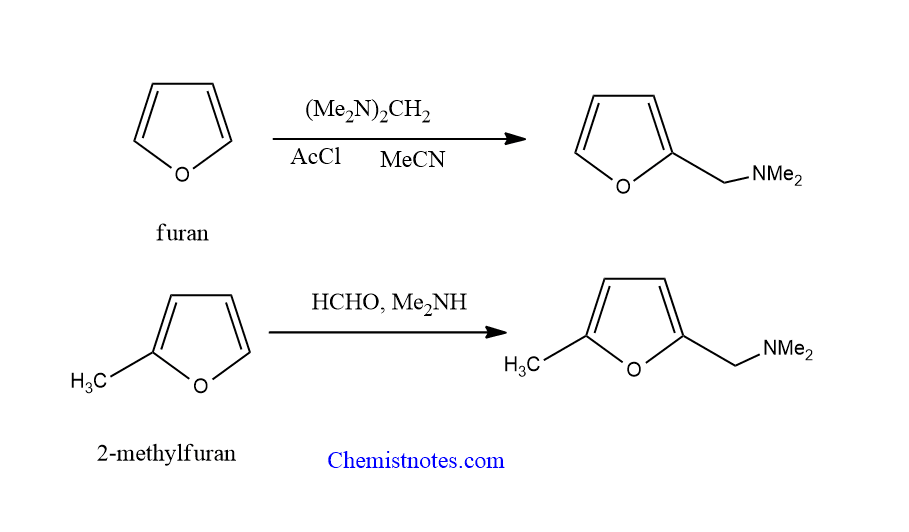
Mannich reaction of furan
Furan undergoes Mannich reaction or Mannich substitution at 2-position only when iminium salt is generated in situ. But mono-alkyl furans reacts in normal condition
Mannich reaction of indole
Indole undergoes a reaction with a mixture of formaldehyde and dimethylamine to give 3-dimethyl aminomethylindole, gramine if the reaction is carried out in high temperature and neutral conditions. Gramine is a very important compound that acts as an intermediate for further synthesis of other useful compounds.
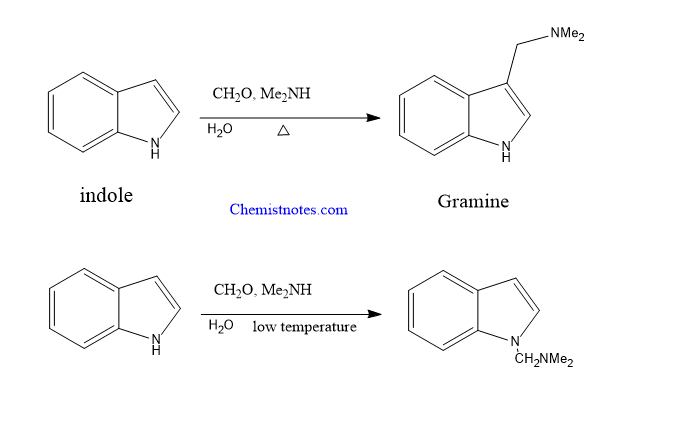
But at low temperature and neutral medium, the substitution takes place at indole N-atom.
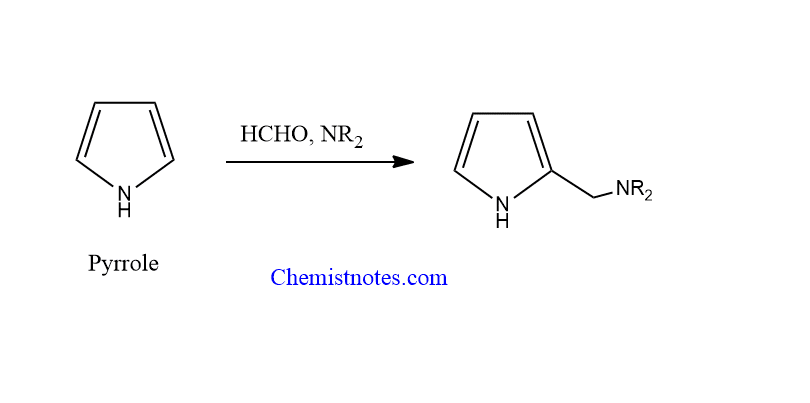
Mannich reaction of pyrrole
Pyrrole can undergo a condensation reaction with imines or iminium ions formed by the reaction between formaldehyde with amines or ammonia. The final product of the reaction is dialkylamino methyl derivatives
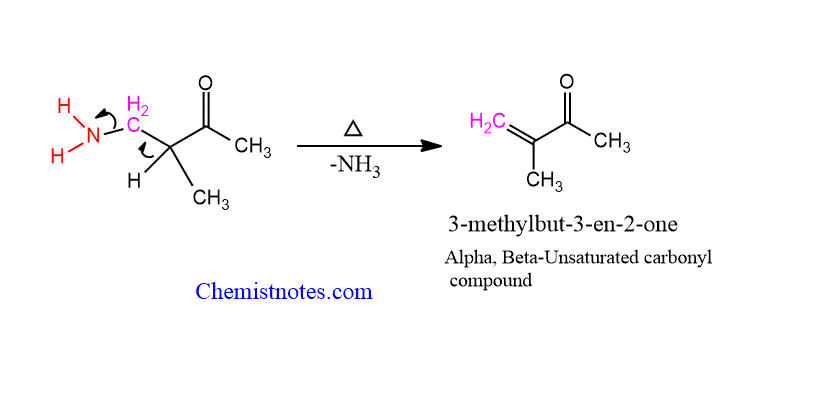
application of mannich reaction
In organic synthesis, Mannich bases are valuable intermediates. When amine is removed, α,β-unsaturated carbonyl molecule is formed.
Another important application of the Mannich base is in alkylation. These are used as alkylating agents. Similarly, the reaction with organolithium and organomagnesium compounds gives β-amino alcohols, which shows pharmacological activities.
Mannich reaction plays an important role in Pharmaceutical chemistry. For example, tropinone and alkaloids can be synthesized by using this reaction.
References:
- Laue T.,Plagens A.,Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005
- Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro, Jr., Name Reaction and Reagents in Organic Synthesis, John Wiley & Sons, 2005
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009






