Table of Contents
ToggleEschenmoser Tanabe fragmentation reaction, examples, mechanism, and application have been discussed below. This reaction was first of all given by Eschenmoser and Tanabe simultaneously in 1967, hence known as the Eschenmoser Tanabe reaction.
Eschenmoser-Tanabe fragmentation
It is the conversion of α,β-epoxyketones to acetylenic aldehydes or ketones using p-toluenesulfonylhydrazine employing bases like pyridine, NaHCO3, or Na2CO3 under moderate circumstances. This reaction is generally known as Eschenmoser fragmentation or Eschenmoser-Tanabe ring cleavage reaction.
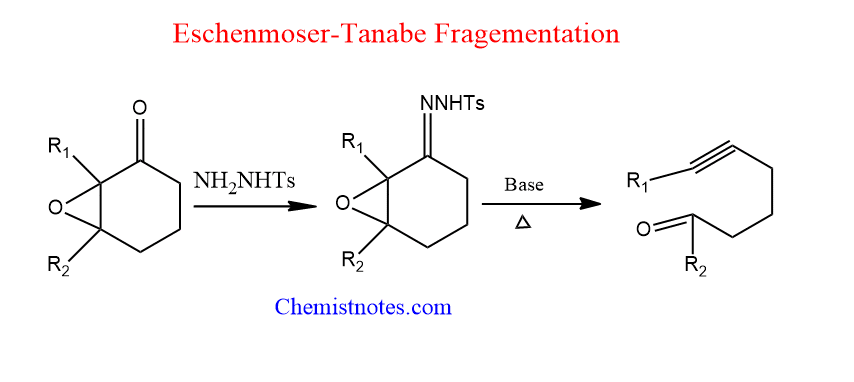
Eschenmoser Tanabe fragmentation mechanism
The mechanism of this reaction completes in the following steps.
Step 1: Formation of Tosylhydrazine derivatives of α,β-epoxyketones.
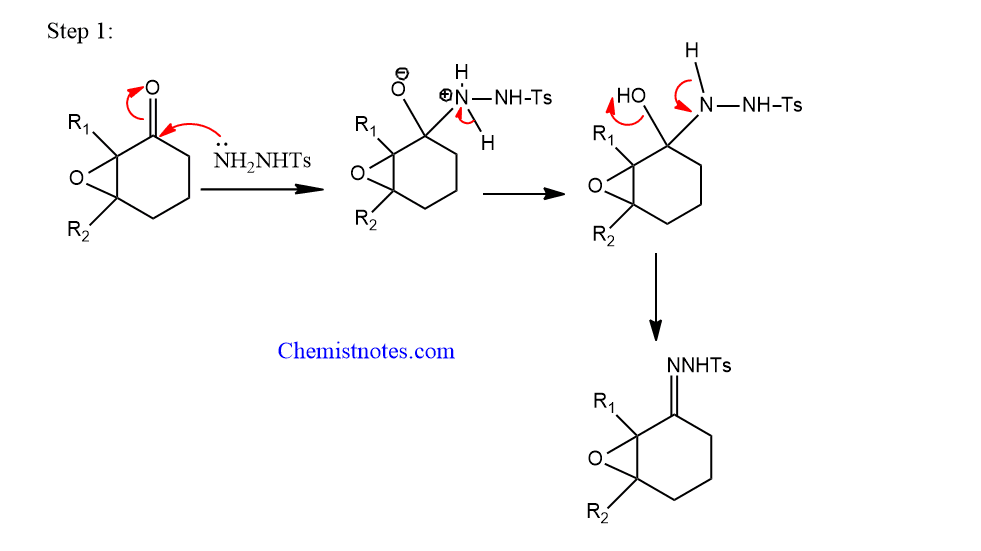
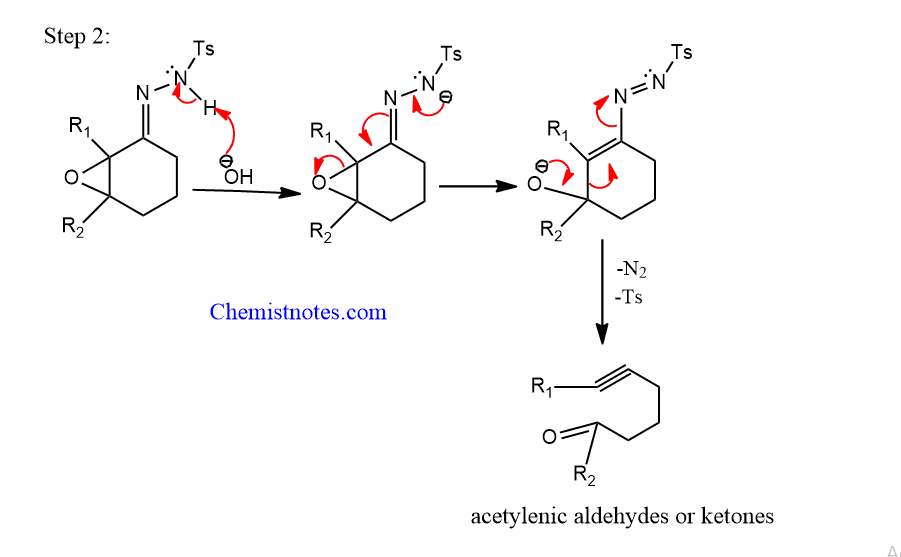
Step 2: The tosyl hydrazone derivatives react with the base to undergo fragmentation.
Application of Eschenmoser Tanabe reaction
Eschenmoser Tanabe fragmentation reaction is used to make alkynes. Moreover, this process has been used to make acetylenic aldehydes and ketones, as well as middle-size cyclic ketones.
References:
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
Please comment if you have any problems related to this topic. If you want to learn more about other reactions such as Mannich reaction, click here.






