Table of Contents
ToggleCorrelation diagram, an approach to rationalizing pericyclic reactions is used to determine the preferred reaction pathways or experimentally observed products for a particular set of molecules. It employs symmetry components as a guiding principle, which must be maintained throughout the reaction.
The orbital symmetries of the reactants and products are taken into account in the correlation diagram. The stabilizing or destabilizing nature of an interaction depends on the relative phases of the two interacting lobes. This method predicts that the antisymmetric (A) orbital of the reactant will correlate with the (A) orbital of the product, and vice versa for the symmetric (S) orbitals of the reactant and products, respectively. However, the (A) orbital of the reactant and the (S) orbital of the product cannot be correlated with one another.
Correlation diagram of interconversion of cyclobutene to butadiene
For symmetry-allowed reaction (bonding overlap), m-symmetric orbitals require a disrotatory mode of rotation while C2-symmetric orbitals require a conrotatory mode of rotation.
Correlation diagram for disrotatory interconversion of cyclobutene to butadiene
Assuming that m-symmetry is maintained throughout the reaction, let’s examine if the disrotatory mode ring-opening of cyclobutene to butadiene is a thermally allowed or photochemically allowed process.
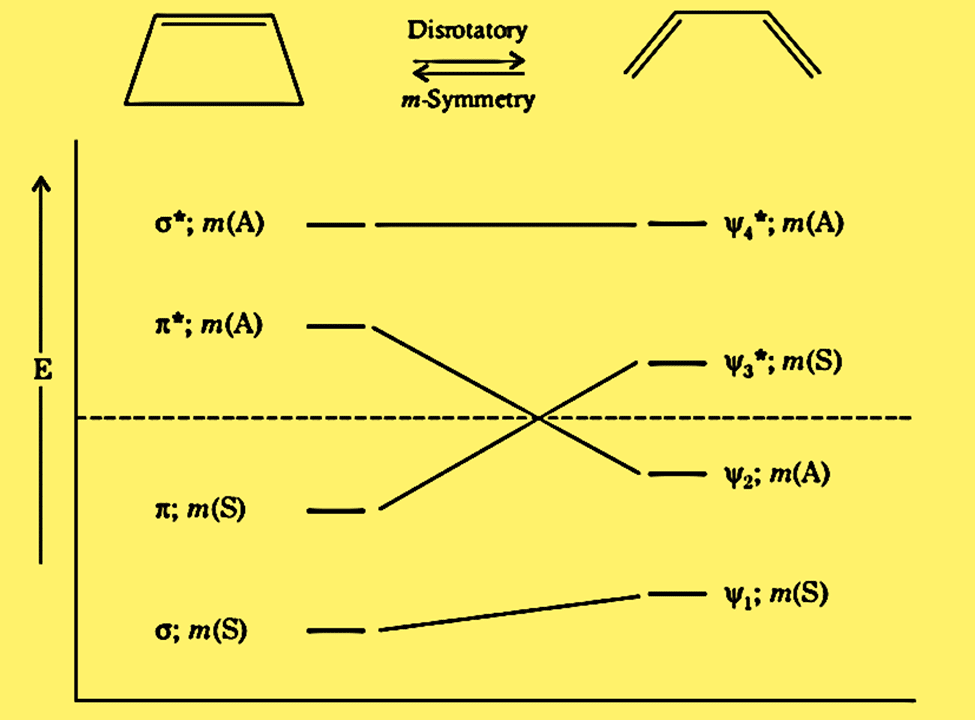
The correlation diagram reveals that this process is thermally prohibited because two electrons in cyclobutene would move to the antibonding orbital (ψ3*) of butadiene. The correlation is observed between the ground state σ orbital of cyclobutene and the ground state ψ1 orbital of butadiene. However, there is no correlation between the ground state π orbital of cyclobutene and the ground state ψ2 orbitals of butadiene. As an alternative, it correlates with the excited state ψ3* and antibonding orbitals.
The correlation diagram demonstrates the correlation between the first excited state of the cyclobutene (σ2, π, π*) correlates with the first excited state of butadiene (ψ12, ψ2, ψ3*). Therefore, photochemically, disrotatory processes in either direction are allowed.
Correlation diagram for conrotatory conversion of cyclobutene to butadiene
The symmetry C2 is maintained throughout the reaction. A correlation diagram of it can be represented as:
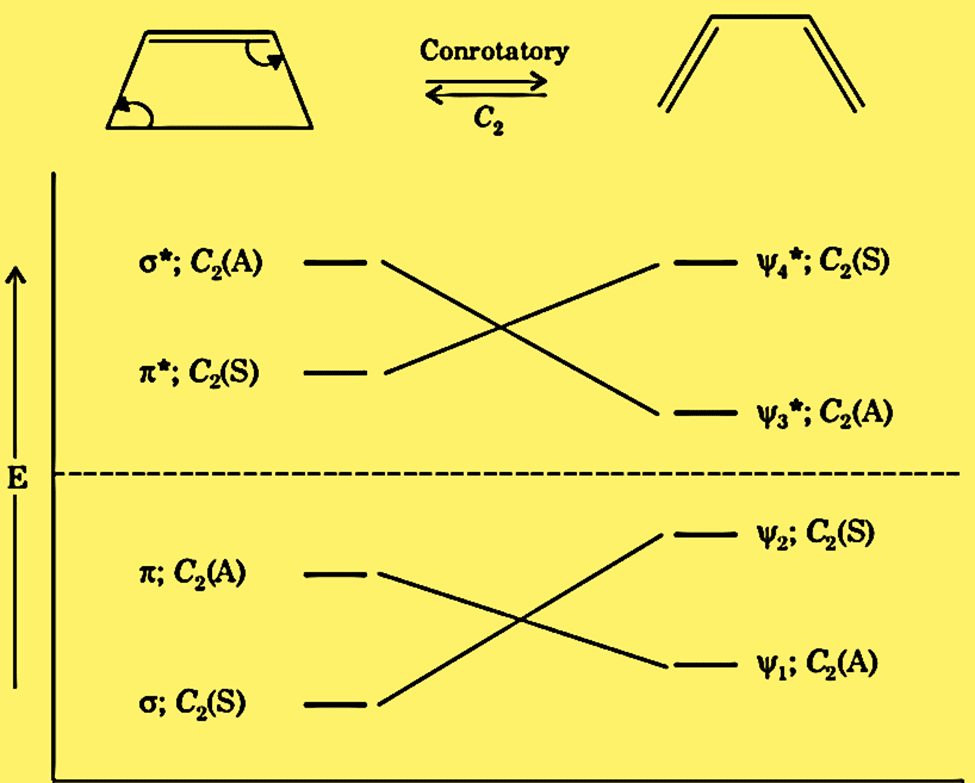
The ground states of butadiene σ2 π2 correlate with the ground state of cyclobutene ψ12 ψ22. Hence, the thermal conrotatory process proceeds in either direction.
Correlation diagram for cycloaddition reaction
Orbital symmetry gives useful predictions about the concerted cycloaddition process.
Correlation diagram for [π2s +π2s] cycloaddition
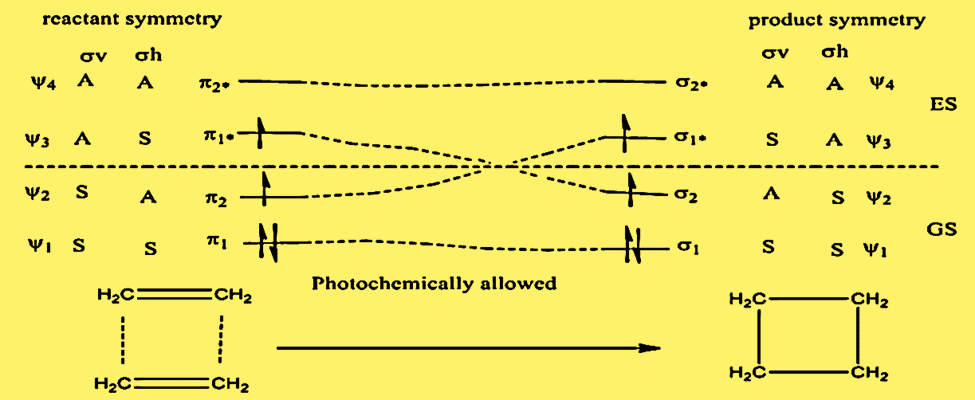
This process is thermally forbidden because the ground state of the interacting system (π12 π22) correlates with an upper excited state of the cyclobutane ( σ12 σ1*2). The initial excited state of interacting ethylene (π12 π2 π1*), however, correlates with the first excited state of cyclobutane ((σ12σ2 σ1*). In account of this, photochemical π2s+π2s cycloaddition is an allowed process.
Correlation diagram for Diels-Alder reaction [π2s +π4s]
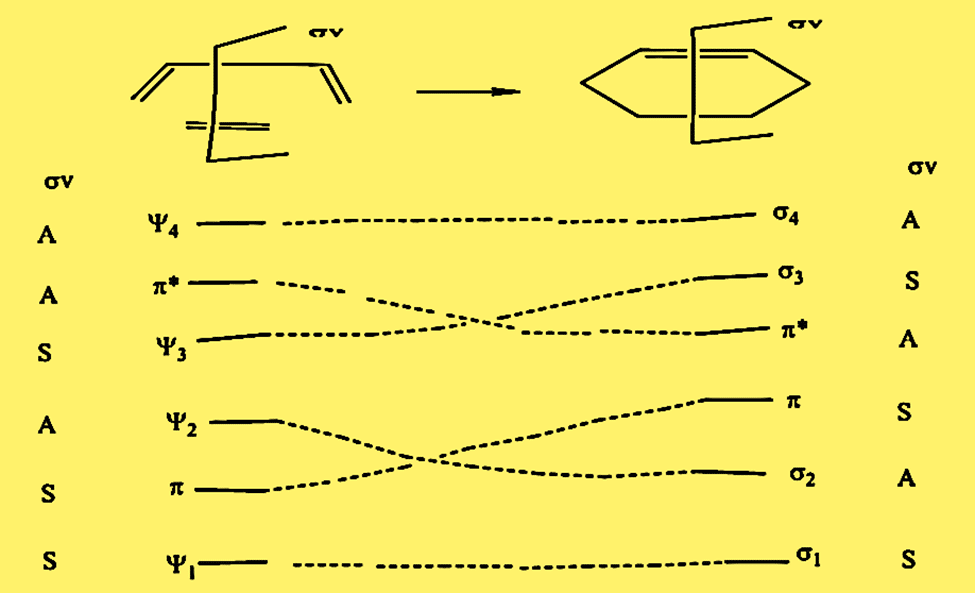
As shown in the diagram, the ground state ψ12 π2 ψ22 correlates with the ground state of another compound (σ12 π2 σ22). This is the reason, the process is thermally allowed, but photochemically forbidden.
Correlation diagram video
References
- R. B. Woodward and R. Hoffmann, Conservation of Orbital symmetry, Verlag Chemie GmbH, Academic Press, 1971.
- Charles DePuy and Orville L. Chapman, Molecular Reaction and Photochemistry, Prentice-Hall, 1972.
- Peter Skyes, A Guide book to mechanism in organic chemistry, sixth edition.






