Table of Contents
ToggleHomologous series, examples, and their characteristics have been discussed in this post. The functional groups present in the organic compounds determine the chemical reactivity and are responsible for the chemical property. But, those parts of organic compounds other than the functional group are responsible for physical properties such as melting points, boiling points, solubility, and so on.
what is homologous series
The organic compound having the same functional group can be arranged in such a way that makes a series in which each member of the series differs by -CH2 units with an adjacent member, is known as homologous series.
- Each members of the homologous series is generally called as Homologues.
- The process or phenomenon of making homologous series is known as Homology.
Characteristics of homologous series
The characteristics of the homologous series are listed below:
- All members of the homologous series are represented by same general formula. For examples: The general formula of alkane series is CnH2n+2 .
- The members of the homologous series are called homologues.
- Two adjacents members(homologues) of the series differ from each other by -CH2 unit in their formula.
- The difference in molecular weight between two adjacent member of series is 14 amu.
- With increasing molecular weight, the physical characteristics of the homologues show an uniform gradation.
- The homologues may be synthesized using almost identical methods and have nearly identical chemical properties.
In most cases, the first member of the series shows different properties in comparison to other members and can be prepared by different methods. Therefore, the second member of the series is truly representative.
Homologous series examples
Every class of organic compounds has homologous series. So, let’s discuss some of the examples.
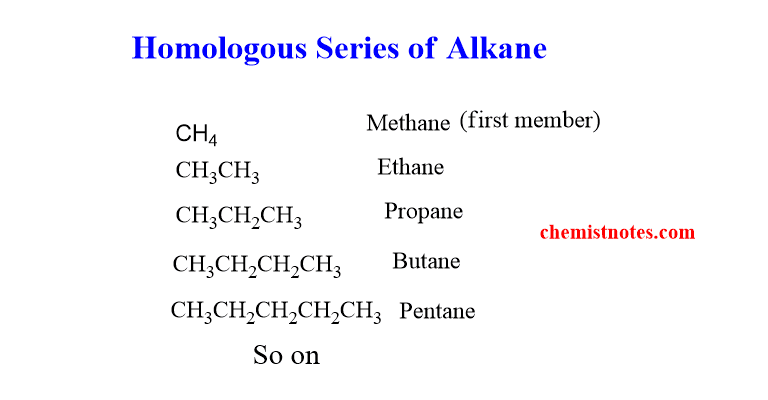
homologous series of alkanes
The series of the alkanes compounds are shown below. The first member of the series is Methane and the second member is Ethane.
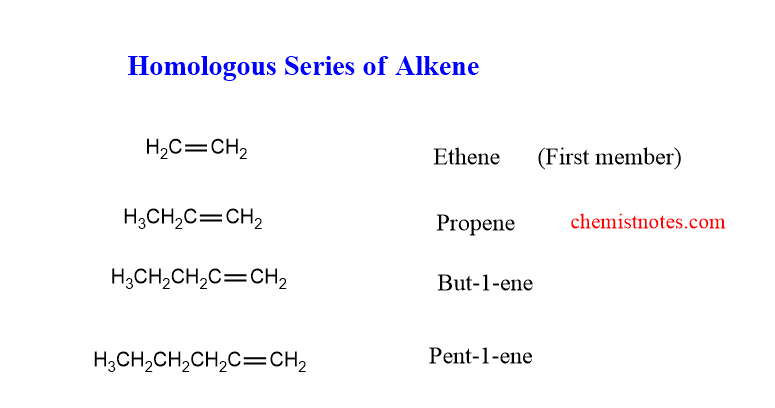
homologous series of alkenes
The homologues of the alkenes are shown below. The first member of the series is Ethene and the second member is Propene.
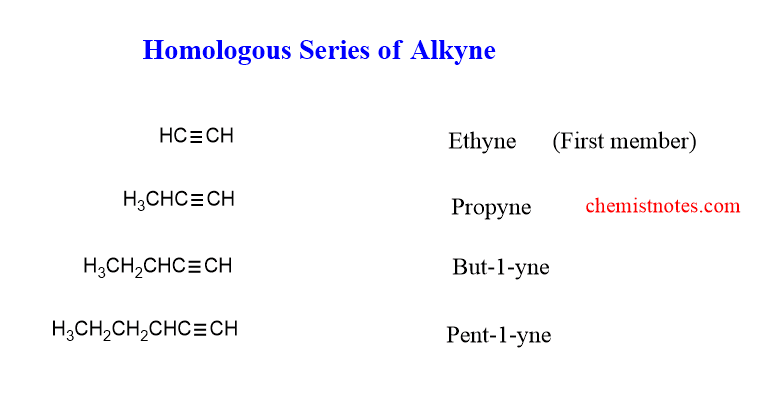
homologous series of alkynes
The homologues of the alkenes are shown below. The first member of the series is Ethyne and the second member is Propyne.
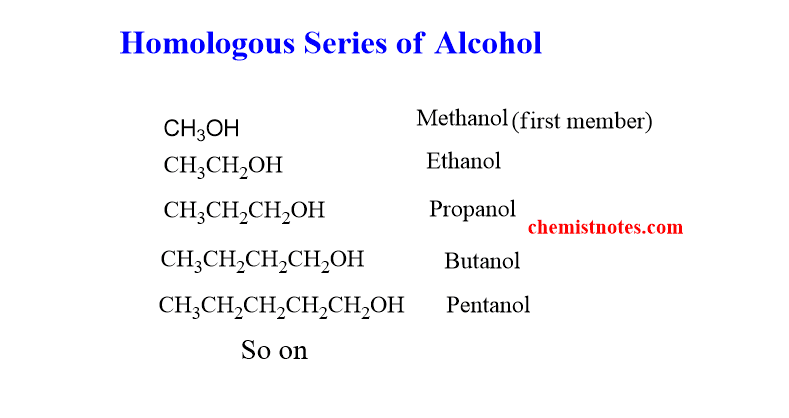
homologous series of alcohol
The series of the alcohol is shown below. The first member of the series is Methanol, and the second member is ethanol.
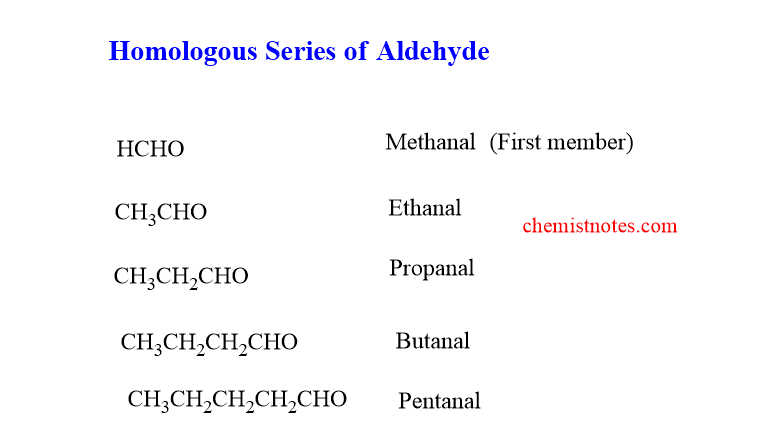
homologous series of aldehyde
The homologous of the aldehyde are shown below. The first member of the series is Methanal and the second member is Ethanal.
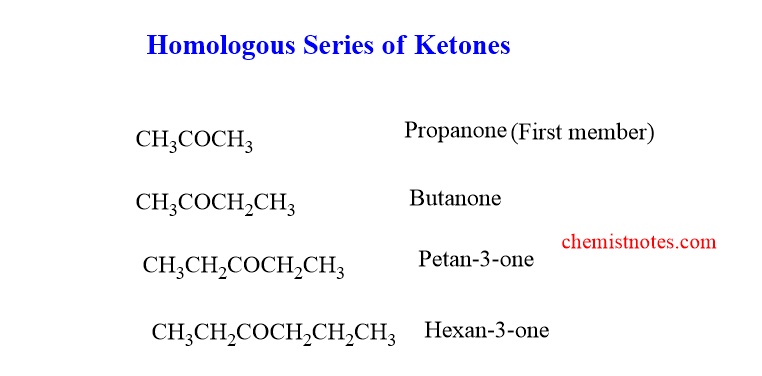
homologous series of ketone
The homologous of the ketone is shown below. The first member of the series is Propanone(acetone) and the second member is Butanone.
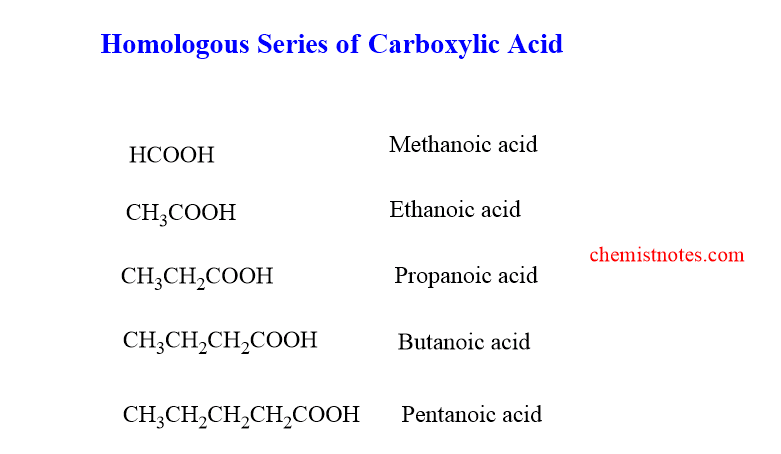
homologous series of carboxylic acid
The homologous of the carboxylic acid are shown below. The first member of the series is Methanoic acid and the second member is Ethanoic acid.
References:
- Bansal, R., A Textbook of Organic Chemistry, 2nd Edition, 1993. Wiley Eastern Ltd., New Delhi, India.
- Harwood, R., Chemistry, 1997. Cambridge University Press, Cambridge, U.K.
- Mahan, B.M., Meyers, R.J., University Chemistry, 4th Edition ISE Reprint, 1998 Addison Wesley Longman, USA.






