Table of Contents
ToggleEthyl acetoacetate (acetoacetic ester) is a useful reagent for the synthesis of methyl ketones. The synthesis is called “acetoacetic ester synthesis of ketones,” and it depends upon the following facts:
- The methylene protons (or ∝-hydrogens) of acetoacetic ester are fairly acidic, and
- Acetoacetic acid (a β-keto acid) decarboxylates readily.
Mechanism of acetoacetic ester synthesis
The synthesis resembles the malonic ester synthesis in several respects, and involves the following steps:
- In the presences of strong base such as sodium ethoxide in absolute alcohol, acetoacetic ester is converted into its salt, known as sodioacetoacetic ester.

- The carbanion thus produced is a nucleophilic and attacks alkyl halide to form an alkylacetoacetic ester
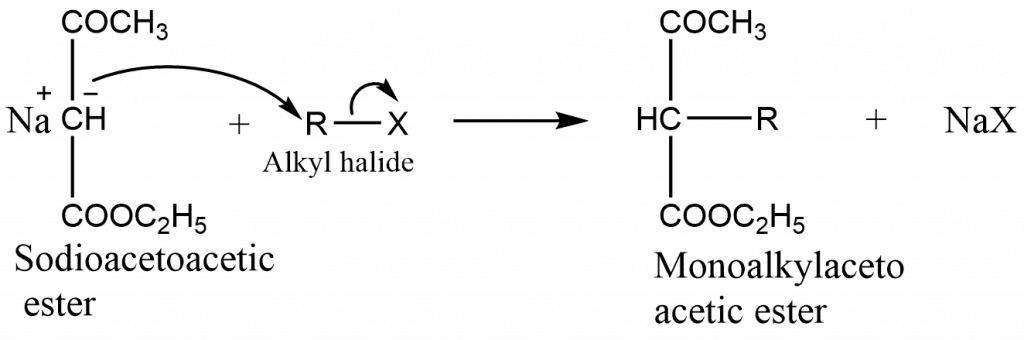
If required, the alkylation can be repeated to produce a dialkylacetoacetic ester.
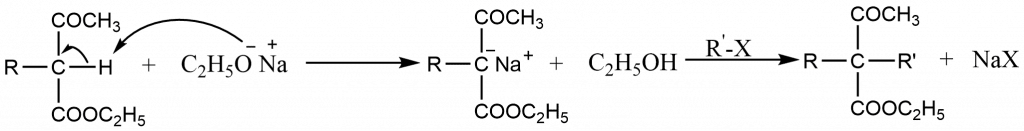
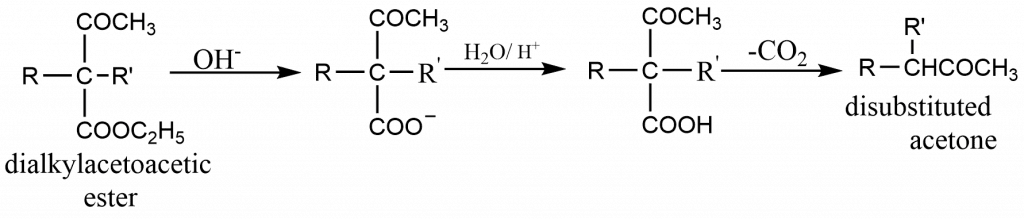
- These mono- or dialkylacetoacetic esters yield the corresponding acids on hydrolysis by dilute aqueous alkali (or by acid). These acids undergo decarboxylation to form ketones. The loss of CO2 occurs more readily than from malonic acid.

Application of acetoacetic ester synthesis
Some examples of acetoacetic ester synthesis
Synthesis of Carboxylic acid
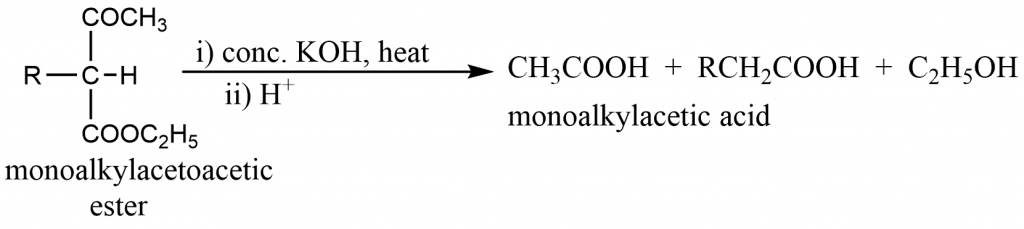
Alkyl derivatives of acetoacetic ester when boiled with conc. KOH solution and acidified yields alkylacetic acid.
Synthesis of dicarboxylic acid
Acetoacetic ester synthesis can be used for the synthesis of dicarboxylic acids. For example, succinic acid may be synthesized by treating sodium salt of the acetoacetic ester with ethyl bromoacetate, and the product is subjected to acid hydrolysis (with conc. KOH).

References
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987






