Table of Contents
ToggleTransuranium elements are the actinide elements that lie beyond uranium, U92 in the long form of the periodic table. These elements are called urenides. Of these, 26 have been identified and named, or they are awaiting confirmation of their discovery. The eleven-actinide elements from Np93 to Lw103 are called transuranic elements, while those with atomic numbers greater than 103 are called transactinoids. The radioactive half-lives of all transuranium elements range from millions of years to a few fractions of a second, making them all unstable and radioactively decaying.
The extraction of these elements is tedious and expensive. All of them have numerous isotopes that emit alpha rays and are created intentionally in nuclear reactors, accelerators, or nuclear weapon explosions.
Transuranium elements list
All elements heavier than plutonium are completely synthetic. The transuranium elements with their atomic number, mass, and mode of decay are listed as:
| Elements | Atomic Number | Atomic Mass | Mode of Decay |
| Neptunium | 93 | 237 | α-decay |
| Plutonium | 94 | 238/239/242/244 (Isotopes) | α-decay |
| Americium | 95 | 241/243 | α-decay |
| Curium | 96 | 242 | α-decay |
| Berkelium | 97 | 249 | β-decay |
| Californium | 98 | 249/252 | α-decay |
| Einsteinium | 99 | 253 | α-decay |
| Fermium | 100 | 257 | α-decay |
| Mendelevium | 101 | 258 | electron capture |
| Nobelium | 102 | 259 | α-decay |
| Lawrencium | 103 | 260 | α-decay |
| Rutherfordium | 104 | 261 | α-decay |
| Dubnium | 105 | 262 | α-decay |
| Seaborgium | 106 | 265 | Spontaneous fisssion |
Synthesis of Transuranium elements
Transuranic elements have been synthesized in the laboratory through nuclear reactions. Following are two types of main nuclear reactions that have been used to synthesize the transuranic elements:
i) By the capture of neutrons
The neutron-proton ratio rises when an element’s nucleus absorbs a neutron (10n). As a result, the host nucleus becomes unstable and loses β-particles leading to a higher atomic number.
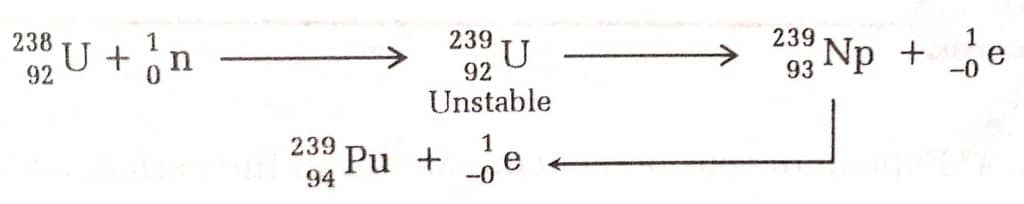
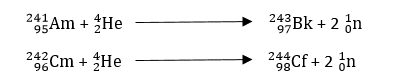
ii) By the capture of unstable nuclei of light elements
The occurrence of these nuclear reactions increases as the atomic number increases. In order to synthesize trans-uranic elements, α-particles have frequently been utilized.
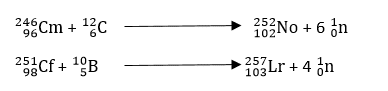
The bombardment of nuclei heavier than particles is necessary to synthesize very heavy trans-uranic elements. An example is the utilization of stripped 126C and 102B nuclei accelerated in a cyclotron for the synthesis of 102NO and 103Lr.
Uses of Transuranium elements
- Plutonium is used as a nuclear fuel in fast breeder reactors. The heat produced as a result of radioactive disintegration is used for generating electricity.
- Neptunium-237 is used for the preparation of Pu-238, which is used as power source in satellites.
- 252Cf as the source of neutron of high flux.
- These elements are widely used in research.
- Used to prepare super heavy elements.
- Used to manufacture lamp filament.
- Used in ceramics technology.
- Used for millatery as well as peaceful purpose.
- Americium is used as smoke detectors.
Transuranium elements Video
FAQs/MCQs
super heavy elements
The elements having atomic number greater than 100 are called super heavy elements.
Trans-fermium elements
The elements placed beyond atomic number (Fermium) 100 are called trans-fermium elements. These are also called super heavy elements.
First transuranium element
Neptunium (Np) is the first transuranium element having atomic number 103.
How are transuranium elements made?
Transition elements are synthesized either by the capture of neutrons or by the capture of unstable nuclei of lighter elements.
What is a transuranium element?
Transuranium elements are those actinide elements which lie beyond uranium, U92 in the long form of the periodic table.
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.






