Table of Contents
ToggleActinides are those elements in which the last electron enters into the anti-penultimate 5f sub-shell. These are the elements from Ac89 to Lw103 that lie in the 7th period of the periodic table and this series of elements are called actinide series. The name actinide is derived from actinium, the very first member of the series. Most of the actinides are synthetic except first four elements of the series; actinium, thorium, protoactinium, and uranium. All the actinides are toxic to humans.
The actinide elements with atomic numbers 93 to 103 (Np93 to Lw103) are called trans-uranium or trans-uranic elements.
Electronic Configuration of Actinides
The general electronic configuration of the Actinide element is
[Xe] 5f0-14 6d0-1 7s2
The electronic configuration demonstrates that the outermost three shells are partially-filled while the remaining inner shells are completely filled. The electronic configuration of the actinides does not follow the simple pattern found in the lanthanides.
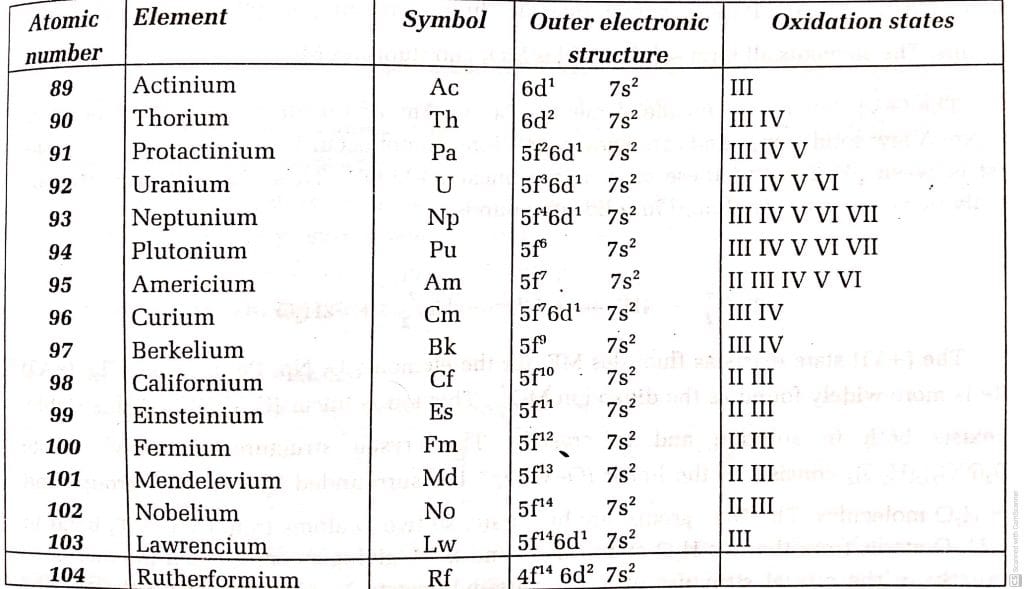
Oxidation states of Actinides
The common oxidation state of actinides elements is +III. Though all the actinides form the +III state like lanthanides, it is not the most stable oxidation state for all of them; especially, for the first four actinides – Th, Pa, U, and NP. The oxidations states of the actinides series are shown in the table.
Characteristics of Actinides
- These elements have the general electronic configuration of 5f0-14 6d0-1 7s2
- They are metallic in nature and easily form their cations.
- The common oxidation state of actinides elements is +III but is not stable for the first four actinides series elements.
- The atomic radii decrease with increasing atomic number (Z) and hence cause actinide contraction.
- Several compounds of the elements are paramagnetic.
- Their ions have characteristic Uv-Visible absorption spectra.
- The actinides form complexes with coordination numbers up to 12.
- The actinides elements like U, Np, Pu, and Am are all quite similar in properties.
- The instability of its nuclei is the primary characteristic of actinides. They spontaneously degrade, mainly emitting alpha particles with some of them split into smaller nuclei by fission.
Actinide Contraction
The ionic radii of the +III actinides are
| Ac3+ | Th3+ | Pa3+ | U3+ | Np3+ | Pu3+ | Am3+ | Cm3+ |
| 1.11Å | 1.08Å | 1.05Å | 1.03Å | 1.01Å | 1.00Å | 0.99Å | 0.98Å |
The Quantum number of the outermost electrons and effective nuclear charge plays a significant role in determining the size of the ion. The outermost electrons are in a completed 6p shell. Since the screening effect of extra electrons in the 5f level does not totally compensate for the increasing nuclear charge, the effective nuclear charge increases with atomic number. The radius contracts as the atomic number increase because the outermost electrons are held tighter and tighter. As a result, the size of the ions contracts from actinium to mendelevium, analogous to the lanthanide contraction. In contrast to the contraction from one lanthanide to the next, this contraction is stronger from one actinide to the next.
Uses of Actinides
- Used as a nuclear fuel.
- Thorium is used in gas mantles and the treatment of cancer.
- Uranium as a nuclear fuel machine.
- Also used as smoke detectors (Americium).
- Additionally, actinium is used as a neutron source, an indicator, and a gamma source.
Actinides Video
FAQ/MCQs
Are actinides radioactive?
The actinides are radioactive in nature and release a huge amount of energy on radioactive decay.
actinide series definition
The elements from actinium (Z = 89) in which the 5f orbitals are being filled up are called actinide series.
actinide periodic table
Actinides are present in the seventh period of the periodic table.
lanthanides and actinides periodic table
Lanthanides are the elements from Ce58 to Lu71 that lie in the 6th period of the periodic table, while actinides are found in the 7th period.
lanthanides and actinides
Lanthanides are those elements in which the last electron enters into the anti-penultimate 4f sub-shell.
Actinides are those elements in which the last electron enters into the anti-penultimate 5f sub-shell.
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.






