Table of Contents
ToggleFajan’s rule for covalency indicates certain conditions which if fulfilled by cation and anion of given ionic molecules, make the ionic bond partially covalent or polar covalent, or purely covalent. No ionic bond is 100% pure ionic because due to the polarisation phenomenon, a certain percentage of covalent character is developed, which is discussed in detail below.
Fajan’s rule
Due to the polarization of anion by cation in ionic molecules or compounds, the covalent character in the compound is introduced. i.e the ionic character between the cation and the anion decreases.
When a cation possesses high polarizing power, it attracts the electron cloud of the anion towards itself more strongly. Thus this causes the overlap of electron charge clouds of cation and anion. This overlap of electron cloud introduces covalent characters. Therefore, the greater the polarizing power of a cation, the greater will be the amount of covalent character produced in the ionic molecules.
The condition which was mentioned by Fajan are called Fajan’s rule, and these conditions are described below.
a.The cation should have a high positive charge on it
Higher is the positive charge on the cation in given ionic molecules, higher will be its polarizing power, and hence greater will be the magnitude of covalent character produced in the ionic bond.
b.The cation should be small in size
Smaller is the cation in its size, greater is its polarizing power, and hence greater in the amount of covalent character produced in the ionic molecules.
c.The cation should have ns2p6d10 configuration
A cation having ns2p6d10 configuration has greater polarizing power than the cation having ns2p6 configuration. Greater polarizing power produces covalent character in the ionic bond.
We can take the example of CuCl and NaCl. CuCl is covalent and NaCl is ionic. This can be explained on the basis of electronic configuration. Cu+ ion has 3s2p6d10 configuration which polarizes Cl–ion to a greater extend than Na+ ion having 2s2p6 configuration.
d.The anion should have high negative charge on it
The greater the magnitude of the negative charge on the anion, the more strongly it gets polarized by a given cation. Hence, the ionic bond induces covalent character we can generalize this as the higher the charge on an anion greater is the extent of the covalent character produced in the ionic bond.
e. The anion should large in size
The larger the size of an anion, the more strongly it is polarized by a given cation, and consequently the covalent character increases.
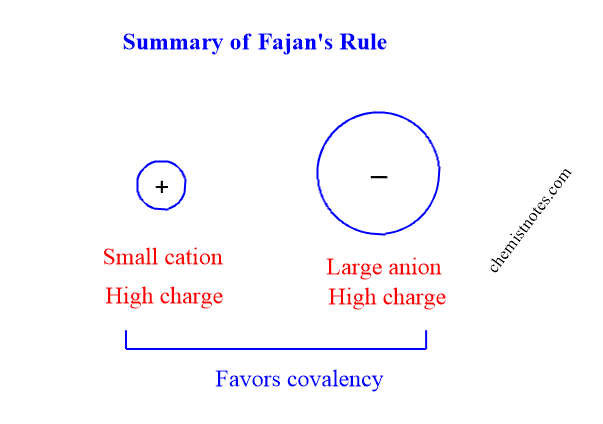
Hence, the formation of covalent bonds in ionic molecules is favored when the cation has a high positive charge, small size, and ns2p6d10 configuration while the anion has a high negative charge and large size.
Application of Fajan’s rule
Fajan’s rule can be used to explain the properties such as covalent character, ionic character, ionic character, melting points, solubility, etc of the ionic compounds.
For example, The melting points of the NaCl, MgCl2, and AlCl3 can be compared by using fajan’s rule.
The magnitude of the positive charge on cation increases from +1 (Na+) to +3 (Al3+). Thus, the polarizing power of cations increases from Na+ to Al3+. Na+<Mg++<Al+++
Due to this, the covalent character increases from NaCl to AlCl3. Therefore, the melting point decreases from NaCl to AlCl3.






