Table of Contents
ToggleThe terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements.
The concept of the nucleus was discovered by Rutheford in his atomic model popularly known as Rutherford’s atomic model, which states that ‘Protons and neutrons, which constitute almost all of the mass of the nuclear atom, are found in the nucleus and lies in the center of the atom‘. Protons with unit positive charge and mass of 1.00758 a.m.u. and neutrons with zero charge and mass of 1.00893 a.m.u. constitute the nucleus. The nucleus of an atom can be represented as:
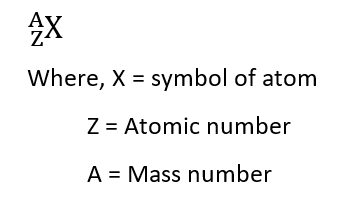
Isotopes
Atoms of the same element having the same atomic number but different mass numbers are called isotopes. It arises due to the difference in the number of neutrons in the nucleus. The chemical properties of isotopes of the elements are the same, however, their physical properties are different.
Isotopes are of two types; stable and radioactive (unstable). Stable isotopes occur in free states without disintegration. On other hand, radioactive isotopes are too unstable and hence split into lighter elements via the emission of rays like α (alpha), β (beta), or γ (gamma).
Example: Isotopes of hydrogen – 11H, 12H, 13H Isotopes of carbon: 612C, 613C, 614C
| Isotopes | Mass number (A) | Number of Protons (Z) | Number of neutrons (A – Z) |
| 11H | 1 | 1 | 0 |
| 12H | 2 | 1 | 1 |
| 13H | 3 | 1 | 2 |
Isobars
Atoms of different elements having different atomic numbers but the same mass numbers are called isobars. The mass number (A) is the representation of nucleons which is the sum of protons and neutrons in the atomic’s nucleus. Isobar possesses different chemical properties, but the same physical properties. Moreover, their electronic configuration also differs.
Example: 1840Ar, 1940K, 1840Ca
| Isotopes | Mass number (A) | Number of Protons (Z) | Number of neutrons (A – Z) |
| 1840Ar | 40 | 18 | 22 |
| 1940K | 40 | 19 | 21 |
| 1840Ca | 40 | 20 | 20 |
Isotones
Atoms of different elements which have the same number of neutrons but different atomic numbers are called isotones. In a general word, the elements must have a same number of protons, but different numbers of protons to be isotones.
Isotones Examples: 614C, 715N, 916O
| Isotopes | Mass number (A) | Number of Protons (Z) | Number of neutrons (A – Z) |
| 614C | 14 | 6 | 8 |
| 715N | 15 | 7 | 8 |
| 916O | 16 | 8 | 8 |
Difference between isotopes and isobars and isotones
| Isotopes | Isobars | Isotones | |
| Definition | Atoms of the same elements with the same atomic number but different mass numbers. | Atoms of different elements with the same mass number but a different atomic number. | Atoms of different elements with the same number of neutrons but different atomic numbers. |
| Atomic number | same | different | different |
| Atomic mass | different | same | different |
| Number of neutrons | different | different | same |
| Examples | 612C, 613C, 614C | 11Na24 12Mg24 | 1737Cl, 1838Ar, 1939K, 2040Ca |
The primary distinction between isotopes, isobars, and isotones can be done based on atomic number, mass, and a number of neutrons. Isotopes have an identical number of protons (atomic number) but different numbers of neutrons and atomic mass, whereas isobars are atoms of different elements with an equal number of atomic mass but different values of the atomic number, and a number of neutrons and isotones are atoms of different elements with the same number of neutrons.
Isotopes, Isobars, and Isotones Video
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






