Table of Contents
ToggleFerrocene is the first metallocene sandwich compound, which was discovered accidentally by TJ Kealy and PL Pauson in 1953. But later, the structure of Ferrocene was determined by G Wilkinson and E O Fischer.
Metallocene is made from two words “Metallo” which stands for transition metal and “Cene” which stands for benzene. Thus, ferrocene is classified under metallocene because the cyclopentadienyl rings react and behave much like normal benzene rings.
Ferrocene
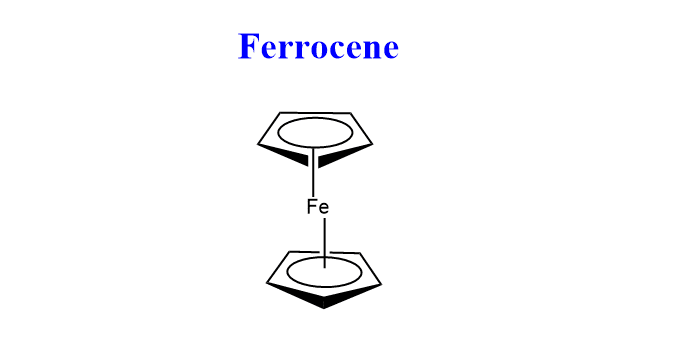
Ferrocene, also known as dicyclopentadienyl iron, has the chemical formula C10H10Fe. The cyclopentadienyl ligands C5H5 bond with the ion in such a way that the iron is present in the center of the complex resulting in a sandwich structure. The oxidation state of iron in this compound is +2. The IUPAC name of ferrocene is bis(η5-cyclopentadienyl)iron
The chemical structure of ferrocene is shown below:
Preparation of Ferrocene
Ferrocene can be synthesized by using the following methods:
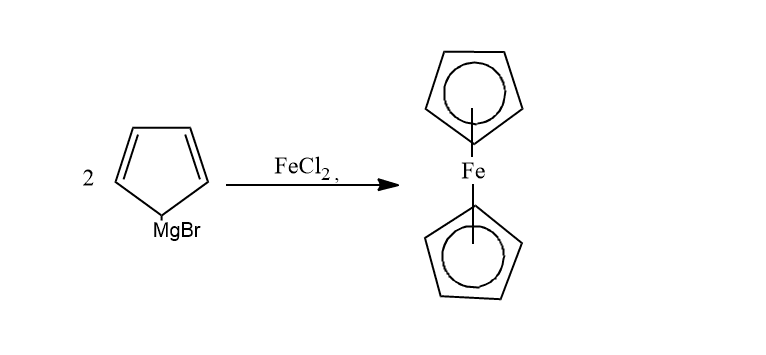
From Grignard reagents
When the Grignard reagent is treated with iron chloride(II), ferrocene is formed.
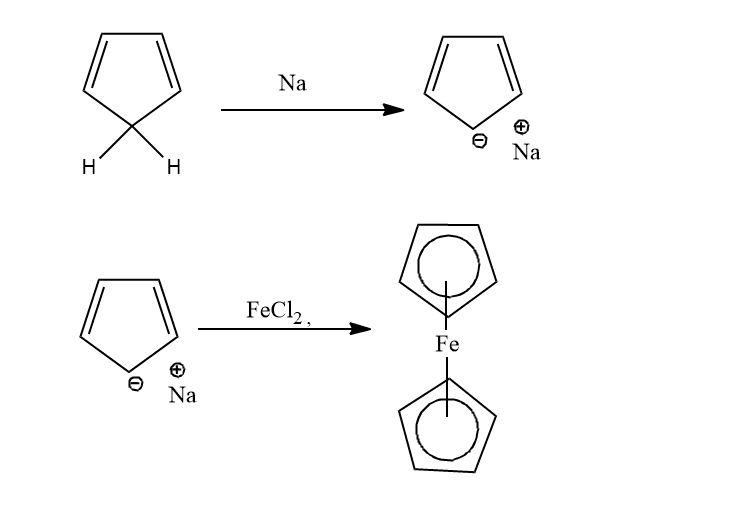
From sodium cyclopentadienide
Cyclopentadiene is treated with sodium metal to form cyclopentadienide, which on reacting with FeCl2 forms ferrocene.
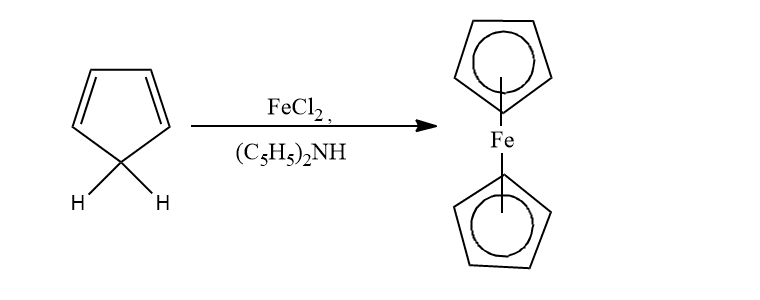
From cyclopentadiene
When cyclopentadiene is treated with iron chloride in the presence of a strong base, ferrocene is obtained.
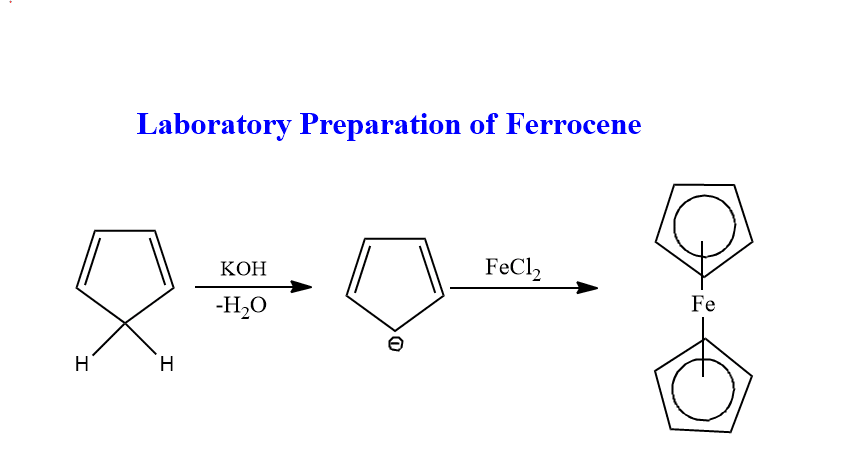
Laboratory method of preparation
Physical properties of ferrocene
Ferrocene is an orange crystalline solid at room temperature having an odor like camphor. It is a stable organometallic compound having melting points of 173-174 °C and boiling points of 249 °C. This compound does not decompose below the temperature of 400 °C. It starts to sublimes at temperatures over 100 °C which makes purification of it easier.
Solubility: It is less soluble in water(0.1 mg/ml) but soluble in organic solvents like DMSO (100 mg/ml) and acetone(10-50 mg/ml).
Chemical properties of ferrocene
Ferrocene can undergo numerous electrophilic reactions faster than benzene. But, it can not undergoes an electrophilic reaction with that electrophile which are strong oxidant such as H2SO4 or HNO3.
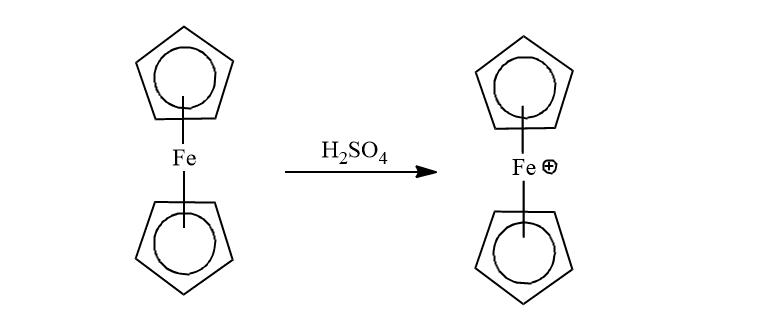
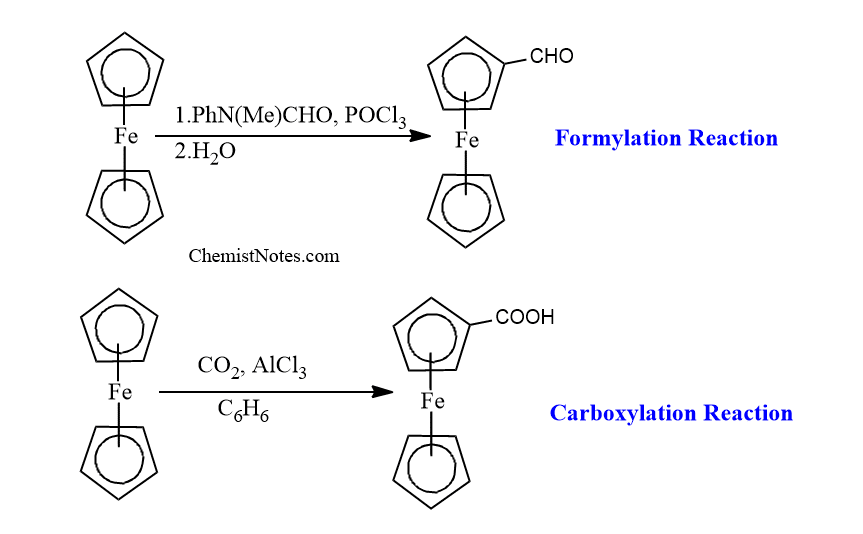
Further, it can also undergo formylation and carboxylation reactions to give only mono-functionalized derivatives because the functional group extensively deactivates the ferrocenyl group.
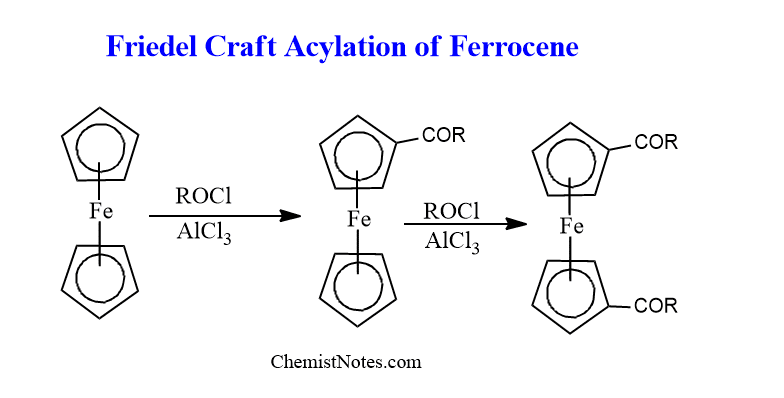
Friedel crafts acylation of ferrocene
Friedel craft acylation reaction can be given by Ferrocene, in which identical reaction also takes place on other rings to give 1,1′-disubstituted derivatives because the substituent has just a little effect on the deactivation of the second Cyclopentadienyl ring(Cp).
Ferrocene structure
Ferrocene has two isoenergetic conformations viz. eclipsed and Staggered conformation. The point group of eclipsed ferrocene is D5h whereas the point group of staggered ferrocene is D5d.
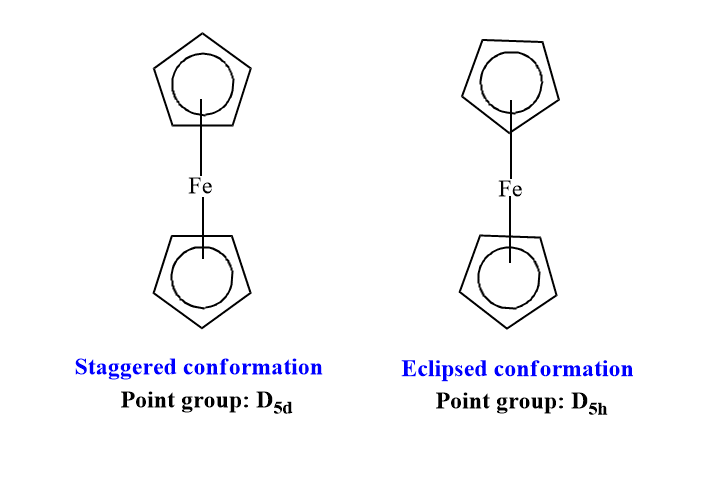
Electronic diffraction and X-ray crystallographic studies show that ferrocene has eclipsed conformation in the gas phase and staggered conformation in the solid phase. The barrier to rotation between the two rings (cyclopentadienyl, Cp) is only 4±1 kJ/mole and this allows the free rotation of rings.
All the carbon-carbon bonds have equal length and the iron is equidistance to all the Cp ring carbons. Moreover, Neutron diffraction also shows that hydrogen atoms on the Cp rings are tilted slightly towards the iron center in ferrocene. Due to this shift, there is a better overlap of the Fe-orbitals and π-orbitals of the Cp rings.
Application of ferrocene
- Ferrocene is mainly used as a catalyst in the industrial world.
- It can be used as a fuel additive due to its catalytic properties and high solubility in less polar organic solvents.
- At high temperatures, it can be used as a lubricant. Therefore, it helps to cut down the mechanical friction, leading to the higher efficiency of the engine.
- Ferrocene and its derivatives can inhibit the degradation of polyethylene by light, thus it is useful in polymerization techniques.
- Moreover, it can increase the thermal stability of polyethylene as well as polypropylene, polyester fiber, plastic, and rubber. Thus it can also act as a protecting agent.
- It is used as an ultraviolet absorber and electron beam sensitizer.
- It is used as a coating in missiles and satellites.
- It is used as a standard in cyclic voltammetry.
- It can be incorporated into the existing drug to improve their activity. For example: Ferrocifen and Ferroquin.
- It also has application in the medical sector as an electrode mediator in the blood glucose level monitor.
Hazards of ferrocene
- It is heat sensitive flammable solid.
- It can cause allergies to the skin, eyes, and respiratory tracts.
- It may be more harmful if it is swallowed.
- If its dust enters the respiratory tract, it may cause respiratory problems.
- Nausea, vomiting, stomach discomfort, and increased salivation are all possible side effects.
- Damage to red blood cells is possible.