Table of Contents
ToggleElectrical properties of solids exhibit a unique range of variation, involving 27 orders of magnitude, that no other physical parameter exhibits. Solids are classified into three kinds based on their electrical conductivity.
- Conductor
- Semiconductor
- Insulator
A solid’s conductivity is one of its distinguishing qualities. Metal has an extremely high electrical conductivity, on the order of 107 ohm-1 m-1. The insulator, on the other hand, has a conductivity of 10-10 to 10-20 ohm-1 m-1. Semiconductors have a conductivity range of 10-6 to 10-4 ohm-1 m-1, which is an intermediate value.
Conductors
The solids which allow the passage of electric current are called conductors. There are two types: metallic conductors and electrolytic conductors.
Metallic conductors are those conductors that undergo chemical changes to allow electricity to flow through them. The conductance of metallic conductors results from the flow of electrons under the influence of an applied voltage. e.g., copper, iron, silver, etc.
Electrolytic conductors are those conductors that undergo chemical changes to allow electricity to flow through them. The movement of ions or other charged particles under the applied field causes conduction in ionic solids. Ionic solids do not carry electricity due to strong electrostatic forces of attraction. When they are in their molten state, or in the form of their molten state, or in the form of their aqueous solution, they conduct electricity well.
Semiconductors
Solids whose conductivity lies between that typical metallic conductors and insulators are called semiconductors. Semiconductors are solids where there is only a small difference in energy, called a bandgap, between the filled valency band of electrons and the conduction band. If cooled to absolute zero, the electrons occupy their lowest possible energy levels, and the conduction band is empty. At normal temperatures, some electrons are thermally excited from the valency band to the conduction band, and hence they can conduct electricity by the passage of electrons at normal temperatures.
Germanium and, to an even greater extent, silicon, are the most important commercial examples of semiconductors. They are not conductors of ordinary temperatures. However, they exhibit appreciable conductivity upon the addition of impurities such as arsenic and boron.
| Compound | Energy gap (kJ mol-1) |
| Ge | 68 |
| Si | 106 |
| Cu2O | 212 |
| ZnO | 328 |
| Diamond | 579 |
Si and Ge at very low temperatures, the valence band is filled and the conduction band is empty. Under these conditions, Si and Ge are both insulators, and cannot carry any electrical current. There are two types of semiconductors.
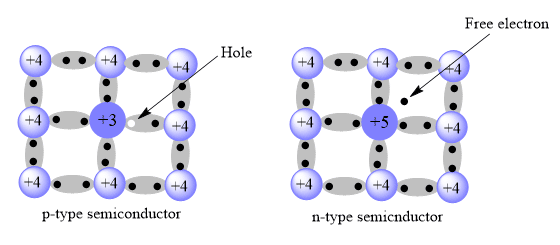
n-type Semiconductor
Semiconductors that are obtained by adding impurities (doping) like arsenic, antimony, or phosphorous having five valence electrons to pure silicon (Si) are called n-type semiconductors.
p-type Semiconductor
The semiconductor which is obtained by adding impurities (doping) like boron, aluminum, indium, gallium, having three valance electrons to the pure silicon is called p-type semiconductors.
Insulator
The solids which do not allow the passage of electric current through them are called insulators. e.g: wood, sulfur phosphorous, rubber, etc.
FAQs
What is a semiconductor?
Solids whose conductivity lies between that of typical metallic conductors and insulators are called semiconductors.
What are the different types of semiconductor?
n-types and p-types semiconductors are two types of semiconductors.






