Table of Contents
ToggleThe inert pair effect, its examples, its cause, and its consequences have been discussed in this blog. Let’s have some knowledge about it.
What is inert pair effect?
The p-block elements with a higher atomic number such as Ga, In, Pb, Sn, As, Bi, etc, show variable oxidation states(generally two) viz. higher oxidation states and lower oxidation states.
- Higher oxidation number:
- These oxidation states are obtained when all the electrons in the valence shell( i.e all the electrons in the ns and np orbitals) are involved in the bond formation.
- These oxidation states are known as group oxidation states and are symbolized by the letter G.
- Simply, higher oxidation states are equal to the group number of the elements or the number of electrons present in the valence shell.
- Lower oxidation states:
- These oxidation states occur when only the electrons in the np orbitals participate in the bond formation.
- As a result, the ns2 electron pair is reluctant to participate in the bond formation. The reluctance of the ns2 electron pair to participate in the bond formation is called the inert pair effect.
- These are equal to the group oxidation state(G) minus two.
Inert pair effect example
As we have stated above, this effect is mainly observed in heavier p-block elements of group IIIA, IVA, and VA. The group elements showing the inert pair effect are listed below:
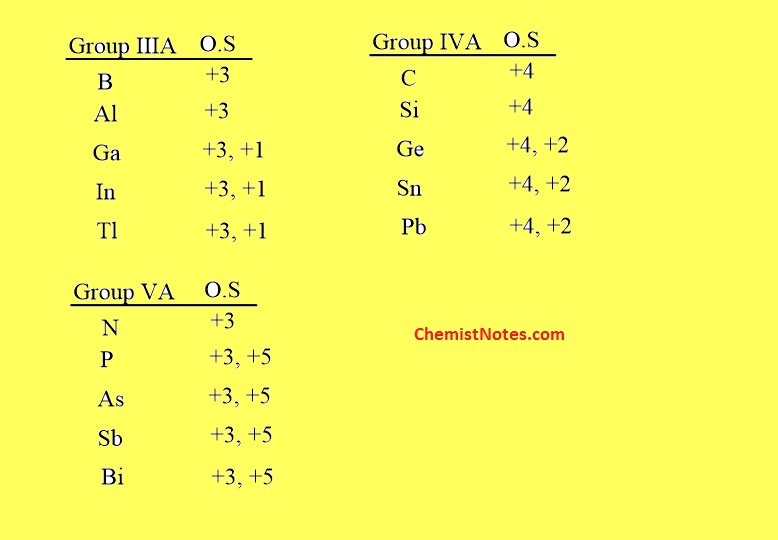
Reason for inert pair effect
For the explanation of the cause of this effect, let us consider a heavy p-block element Gallium(Ga) whose valence shell electronic configuration is ns2np1.
One np electron is farther from the nucleus than two ns electrons. As a result, the single np1 electron may be readily removed or excited to higher orbitals than ns2 electrons. As a result, the two ns electrons stay paired and do not participate in bond formation. Thus, Galium creates Ga+ more easily than Ga3+. It means Ga prefers to exist in the +1 oxidation state( lower oxidation state) and shows reluctance to exist in the +4 oxidation state(higher oxidation state).
Variation of inert pair effect in a group
As we progress down the group, the inert pair effect grows stronger and more powerful. Therefore, this effect is absent in the elements situated at the beginning of a group and becomes prominent in the heavier elements of the groups. It means, when we move down the group, the ns2 electron pair present in the cations having lower oxidation states becomes more and more inert and hence tends to remain paired. As we progress down the group, the ns2 electron pair is not readily lost to create the cation with a higher oxidation state due to its stability.
Consequences of inert pair effect
This effect can explain the relative stability of the two types of oxidation states displayed by heavier elements of groups IIIA, IVA, and VA. Let’s explain the relative stability of the +1 and +3 oxidation states of the elements of groups IIIA.
- The inert pair effect is absent in the lighter elements of Group IIIA (B and Al) because both elements lose all three ns2p1 electrons to produce B+3 and Al+3 cations. But due to its small size and high electron density, B forms covalent compounds and hence does not give B+3 ions. Thus, B and Al show +3 oxidation states(group oxidation state).
- The heavier elements, Ga, In, and Tl, exhibit the inert pair effect and have +1 (lower oxidation state) and +3 (higher oxidation state). The 4s2 electron pair in Ga+ ion (Ga+= 4s2p0) is neither stable nor inert, and hence electrons are rapidly lost to transform these monovalent cations into more stable trivalent cations Ga+3. Being less stable, Ga+ (+1) is easily oxidized to a more stable Ga+3 ion.
Similarly, the relative stability of the +2 and +4 oxidation states of the elements of group IVA and the relative stability of the +3 and +5 oxidation states of the elements of group VA can be explained.
In conclusion, because of the inert pair effect, the lighter elements of groups IIIA, IVA, and VA prefer to display higher oxidation states that are equivalent to group oxidation states and are reluctant to exhibit lower oxidation states. The heavier elements in these groups, namely Tl, Pb, and Bi, tend to display lower oxidation states and are unwilling to exhibit higher oxidation states.
FAQs/MCQ:
Which elements show inert pair effect?
Heavier p-block elements show an inert pair effect.







3 Responses
Wonderful explanation so lucid and articulate as even a person like me from a commerce background was able to comprehend the concept with such a great ease.
Thanks for the fantastic explanation. It really spelled magic while i was helping my daughter to familiarize with Group 13 elements. I will continue to explore more stuff moving further. It would be great if you can provide similar explanation on the screening effect.
Sure, we will try our best.
Well we have a content on screening effect topic. Hope you gonna like it. Thank you