Table of Contents
ToggleNephelauxetic effect in inorganic chemistry is a popular term. The nephelauxetic effect gives indirect evidence that electrons are shared between the ligands and the central metal ion i.e metal-ligand orbital overlap. This effect is also known as the cloud expanding effect.
Nephelauxetic effect
The experimental data is not completely fitted if the energy level for transition metal ions with 2 to 8-d electrons is calculated under the assumption that the separations between various Russell- Saunders (LS) states are exactly the same in the complexed ion as in the free gaseous ion. However, by assuming that the separations between the Russel Saunder’s states are smaller in the complexed ion than in the free ion, the fitting can be improved.
The electron-electron repulsion in complexes is found to be less than that of a free metal ion. In the dn configuration, the decrease in energy separation between the Russel-Saunders states indicates that the d-electrons cloud has expanded in the complex, increasing the mean distance between the d-electrons and decreasing interelectronic repulsion.
The enlargement of the d-electron cloud is thought to be due to the orbital overlap between the metal ion and the ligand, which allows d-electrons to escape from the metal ion to some extent. This effect is the result of ligands expanding the d-electron cloud. This effect of ligands in expanding the d-electron cloud is called the nephelauxetic effect.
Therefore, this effect can be defined as the effect of ligands in expanding the d-electron clouds in the metal-ligand complex.
Nephelauxetic series
The common ligands can be arranged in order of their ability to cause cloud expansion. The common ligands can be arranged in the order of decreasing electron-electron repulsion. This series is known as the nephelauxetic series.
The nephelauxetic series of ligands is given as:
F–>H2O > Urea> NH3> En ≈ Ox> NCS–> Cl–≈ CN–> Br–> N3–> I–
Where, En=Ethylenediamine, and Ox=Oxalate
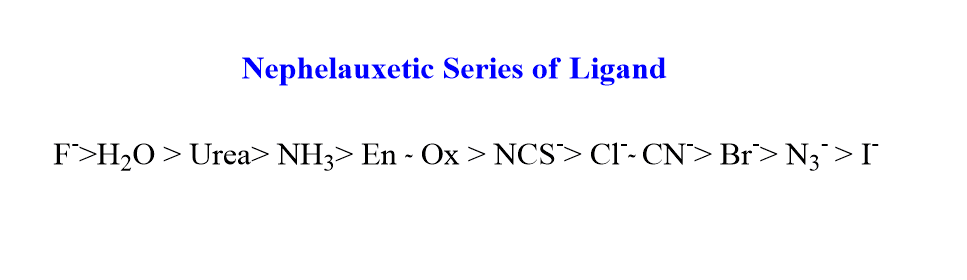
Nephelauxetic ratio
The nephelauxetic ratio is used to express the magnitude of the nephelauxetic effect. It is denoted by beta (β). It is defined as the ratio of a metal ion’s interelectronic repulsion parameter in complex to its value in a gaseous free metal ion.
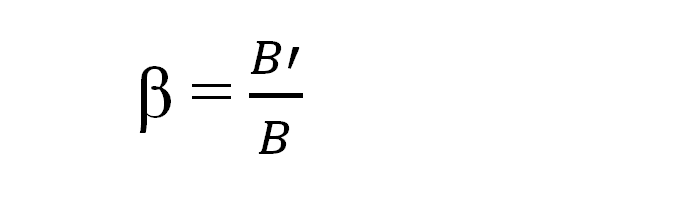
Where B= electron repulsion parameter of complex and B’=electron repulsion parameter of free metal ion in gaseous form.
Spectrochemical and nephelauxetic series
Spectrochemical series is the experimentally determined series on the basis of the strength of ligand field for the splitting of d-orbital of metal ion of complexes. But the nephelauxetic series is the arrangement of ligands in series on the basis of their strength in the expanding d-electron cloud.






