Table of Contents
ToggleNonstoichiometric or Berthollide compounds exist over a range of chemical compositions. Nonstoichiometric defects occur when the ratio of one type of atom to another type of atom, or the ratio of cations to anions, deviates from whatever is stated in the chemical formula. The stoichiometry of the compound is modified by this imperfection. If the ratio of atoms is not exactly 1:1, there must be either an excess of metal ions or a deficiency of metal ions. Electrical neutrality is maintained either by having extra electrons in the structure or by changing the charge on some of the metal ions. This makes the structure irregular in some way, i.e. it contains defects.
Metal excess
This may occur in two different way
F-Centres (colour centers)
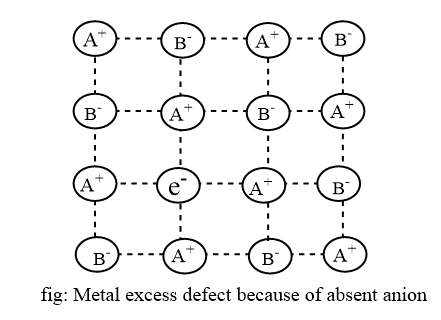
In this case, an anion is absent from its normal lattice site and holes created by it is occupied by an electron are called F-centres, which is principally responsible for the colour by absorbing light in the visible region. This defect is found in Schottky defect-forming compound.
If a colourless stoichiometric NaCl crystal is heated in the presence of sodium and potassium vapour and rapidly quenched, extra sodium or potassium atoms enter into the host lattice. They get ionized as Na+ + e– or K+ + e– where, Na+ or K+ ion occupies a normal cation site in crystal structure but e– is trapped in anion vacancy and NaCl becomes yellow.
Interstitial ions and electrons
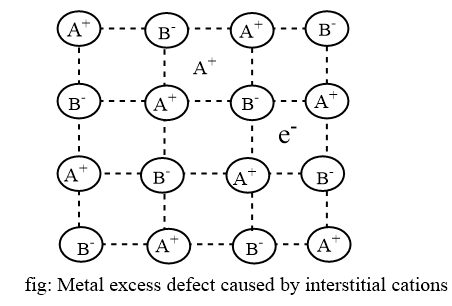
This defect occurs when a cation occupies an interstitial site and an electron is included in the interstitial position for electrical neutrality. It is common in crystals with frenkel defects. They of defect generally observed in ZnO, Fe2O3, CdO etc.
Metal Deficiency Defects
This defects may occur in two ways. Both require variable oxidation state of metal and might therefore be expected with the transition metals.
Positive ions absent
This defect occurs when one or more cations are missing from their sites, resulting in cation vacancies. To maintain charge balance, one or more neighboring cations acquire extra positive charge and are thus transformed into cations with greater oxidation states. This defect is present in ionic crystals where the positive ions have a variable oxidation state. For example; FeO, NiO, FeS etc.
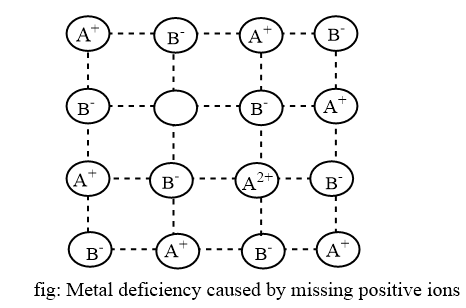
Crystal having metal deficient defects are semi conductors. The conductivity is due to the movement of an electron from one cation to the other cation. When an electron moves from one cation, is is changed into a cation with higher positive oxidation state. The movement of cation is also called the movement of positive hole and the substance are called p-type semiconductor.
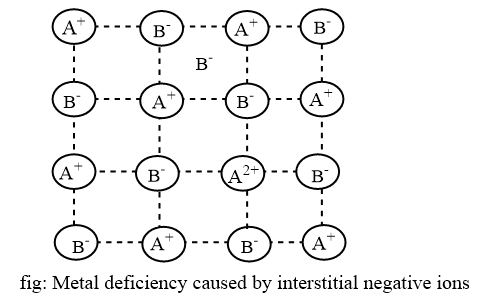
Extra interstitial negative ions
An extra negative ions present in an interstitial position and to maintain charge balance, a nearby cation acquire extra positive charge. However, since negative ions are usually large, it would be difficult to fit them intoo interstitial position. No examples of crystals containing such as negative interstitial ions are known at present.
Difference Between Stoichiometric Defect and Nonstoichiometric Defect
| Stoichiometric Defect | Nonstoichiometric Defect |
| Stoichiometric defects are those that do not affect a compound’s stoichiometry. | Non-stoichiometric defects are defects in crystal structures that disturb the stoichiometry of the compounds. |
| They do not affect the stoichiometry of the compound. | They change the stoichiometry of the compound. |
| There are several typesof stoichiometric defect such as, interstitial defects, schottky defects, and Frenkel defects. | Metal excess defects and metal deficiency defects are two types of Nonstoichiometric effect. |
FAQs
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.






