Table of Contents
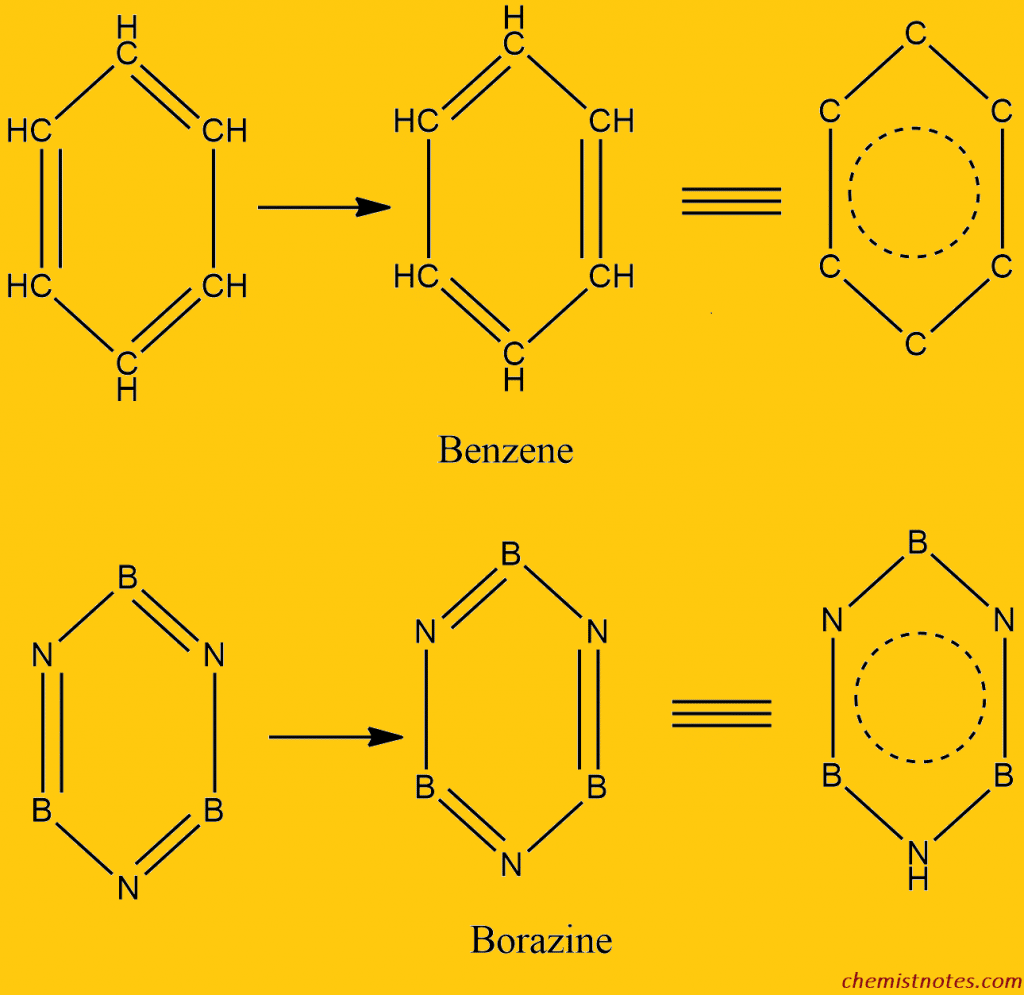
ToggleBorazine or Borazole (B3N3H6) is a heteropolymer of boron and nitrogen having a hexagonal structure with B and N in an alternate position. It is also called “inorganic benzene” because its structure resembles that of benzene.
B-N polymerization takes place by
- Ring-opening reaction of various borazines.
- joining rings of borazines in some fashion to form linear molecules.
Preparation of Borazine
- By stocks method
In this method, diborane (B2H6) and NH3 are treated in a 1:2 molar ratio to get the adduct B2H6. 2NH3, which then gets decomposed by heating a closed tube at 200o C to give borazine.
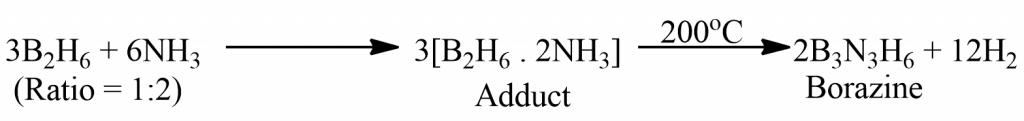
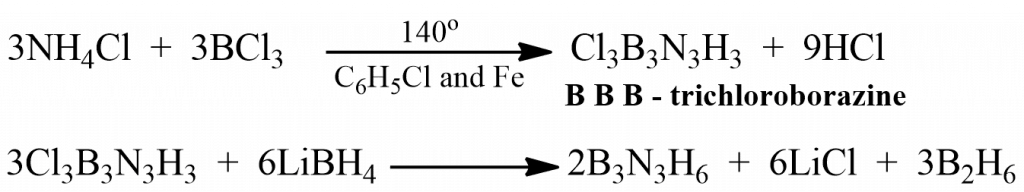
- By heating BCl3 with NH4Cl
BCl3 on heating with NH4Cl in chlorobenzene in presence of Fe, Ni, or Co (as a catalyst) at about 140oC gives B, B, B- trichloroborazine, which on being reduced by LiBH4 in polyethylene, gives borazine.
- By heating a mixture of LiBH4 and NH4Cl in a vacuum at 230oC. It is a laboratory method of preparation.

- Borazine and substituted borazines are synthesized by the following reactions.

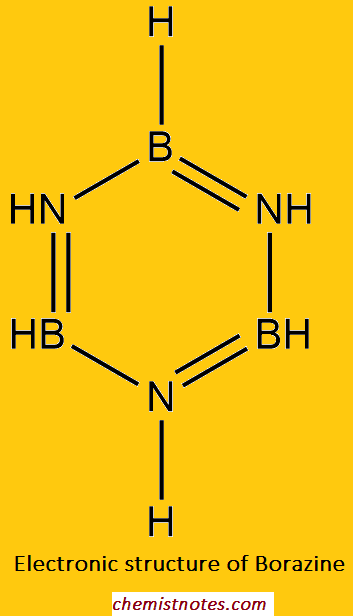
Sturucture of Borazole (Borazine)
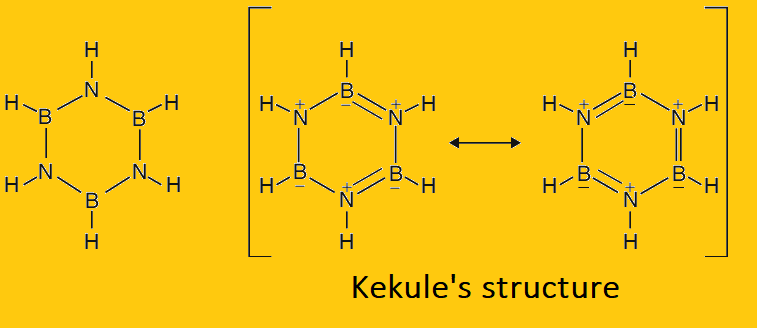
X-ray crystallography has shown that B and N atoms in borazine are arranged alternatively in a planar, hexagonal ring in which the B-N bond length is 1.44 Ao, which is similar to the C-C bond length (1.42Ao) in benzene. The bonding in borazine has been described in terms of resonance between the single-bonded tri amino-triborane ring and Kekule’s structure, in which double bonds are formed due to the participation of the lone pairs on N-atoms in B-N bonds. This results in a formal negative charge on boron and a formal positive charge on the N-atom.
The molecular orbital description of borazine takes into consideration the formation of sigma and pi bonds in the ring.
Similarities of Borazine with Benzene
- Borazine is isoelectronic with benzene. Electron diffraction studies have shown that borazine has a cyclic structure resembling that of benzene with alternating ‘B’ and ‘N’ atoms. Like benzene, there is a system of conjugated double and single bonds. It involves resonance between two Kekule configurations similar to the Kekule structure of benzene.
- In physical properties (M.Pt, B.Pt, density, etc) borazine is indeed a close analogy of benzene.
- The ring structure of borazine is related to the layer structure of boron nitrite like the structure of benzene is related to the layer structure of graphite.
Due to these similarities, Borazine is called inorganic Benzene.
Difference between Borazine and Benzene
The main chemical properties of borazine are quite different from those of benzene.
1. Addition reaction
Borazine is considerably more reactive than benzene, and addition reactions occur quite readily. The difference in chemical behavior towards the polar reagent is probably due to the polarity of B-N bonds in benzene.
Borazine readily undergoes an addition reaction, whereas benzene does not:
Reaction with HCl
One molecule of borazine adds three molecules of HCl in the cold to give hydrogen chloride derivatives. The more electronegative Cl atom of the HCl molecule is attached to B since the B-atom is less electronegative than the N-atom in the B-N bond. When hydrogen chloride derivatives are heated at 50-100 oC, they lose three H2 molecules to give B, B’, B”-trichloro borazine.
Bromination reaction
On bromination, borazine gives B-tribromo-N-tribromo borazine, which on heating to 60 oC losses three molecules of HBr and forms B-tribromo borazide.
On the other hand, benzene with bromine in presence of FeBr3 yields monobromo benzene(substitution)
2. Hydrolysis reaction
Borazine very slowly hydrolyzes to hydrogen, boric acid, and ammonia. On the other hand, benzene doesn’t undergo a hydrolysis reaction.
3. Hydrogenation
Hydrogenation of borazine results in polymeric materials of indefinite composition but benzene gives cyclohexane.
Properties of Borazine
- Colorless mobile ligand liquid with an aromatic smell.
- The melting point is -58oC and the boiling point is 55oC.
- It forms an addition compound with HCl. On heating this compound, BB’B”- trichloroborazole is produced, and by condensing this compound with ammonia, it will give a polymer.
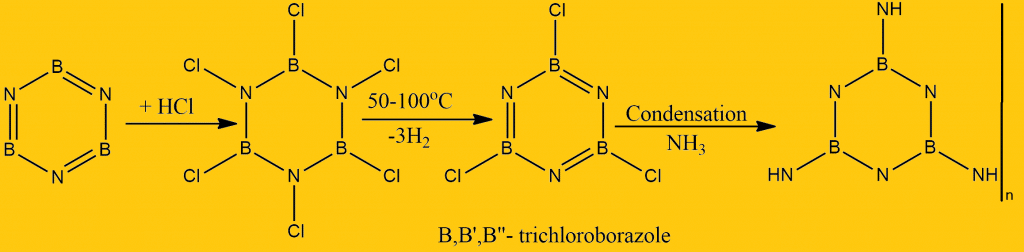
- Borazole is reported to polymerize slowly on standing at room temperature, evidently forming a fused ring structure.






