Table of Contents
ToggleBiological oxygen demand (BOD) has been a standardized measurement of the amount of oxygen that would be required by microorganisms to cause the decomposition of certain organic and inorganic matter in the water. Hence, the amount of oxygen required by microorganisms to oxidize organic wastes aerobically is called the BOD. Water contains molecular oxygen, which is either a result of photosynthesis in aquatic plants or atmospheric oxygen in the dissolved state. The greater the BOD of a body of water or water sample, the more it is polluted.
The measurement is done under standardized conditions (e.g., at 20oC and 5 days to allow the decomposition to take place). The result is called the 5-day BOD and is expressed in milligrams of oxygen per liter of water. BOD is not a pollutant but an indicator. For “excellent” drinking water, the 5-day BOD, on a monthly average, should be in the range of 0.75-1.5 milligrams/liter. BOD values are important when they indicate that the oxygen supply dissolved in water will be so low that described fish no longer can survive or when they signify that conditions for the propagation of dangerous bacteria exist. When the BOD of a water body increases significantly, aquatic life is adversely affected.
Calculation of BOD
BOD is calculated by measuring the oxygen consumed (decrease in dissolved oxygen) by bacteria and chemical action in a closed sample of water maintained at 20 oC for five days. BOD is expressed in milligrams per liter. The BOD values may range from 3 to 10, depending on the quality of the water. Clean water samples have low BOD values, whereas polluted water samples have high BOD values.
Principle
An aliquot of the water sample is maintained in an incubator at 20oC for five days in a closed bottle, without allowing air to enter; during this time the water sample is assumed to be established, that is the bacterial decomposition gets completed. Measuring the dissolved oxygen in the water sample before and after incubation would indicate the amount of oxygen used for stabilizing the water.
A water sample with a low BOD can be straightway used for BOD determination. But a water sample with high BOD must be diluted and pretreated before the determination of BOD.
Calculation
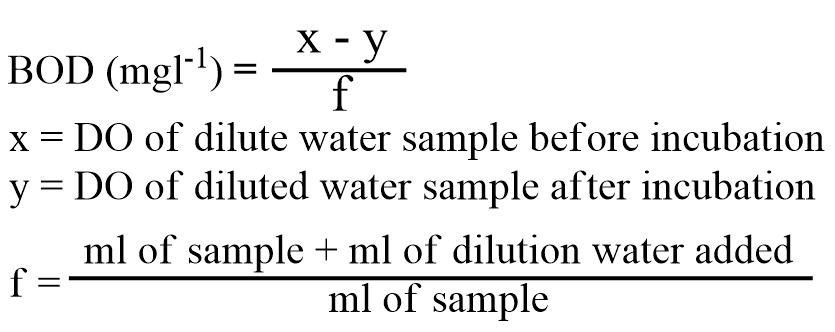
- Below 1 mg/L = natural water quality
- 2-8 mg/L = moderately polluted water
- Above 8mg/L = severely polluted water
Importance of BOD
- In secondary sewage treatment or biological sewage treatment, biochemical oxygen demand (BOD) is used.
- Biological oxygen demand discharged wastewater will have on our environment.
- BOD is widely utilized in wastewater treatment because the decomposition of organic waste by microorganisms is a common treatment method.
- The results of the BOD test are used to design wastewater treatment plants.
FAQs
What is biological oxygen demand (BOD)?
The amount of oxygen required by microorganisms to oxidize organic wastes aerobically is called the BOD.






