Table of Contents
ToggleThere were many periodic tables developed but the Mendeleev’s periodic table has been the most important . In 1869 after just 5 years John Newlands put forward his Law of Octaves, a Russian chemist called Dmitri Ivanovich Mendeleev published a periodic table.
Mendeleev’s periodic rule states, “The physical and the chemical properties of elements are a periodic function of their atomic masses.”
Mendeleev also arranged the elements known at the time in order of relative atomic mass, but he did some other things that made his table much more successful. On the basis of this periodic law Mendeleev constructed a periodic table in such a way that the element were arranged horizontally in order of their increasing atomic masses.
In Mendeleev’s Periodic Table elements were arranged by increasing atomic masses.
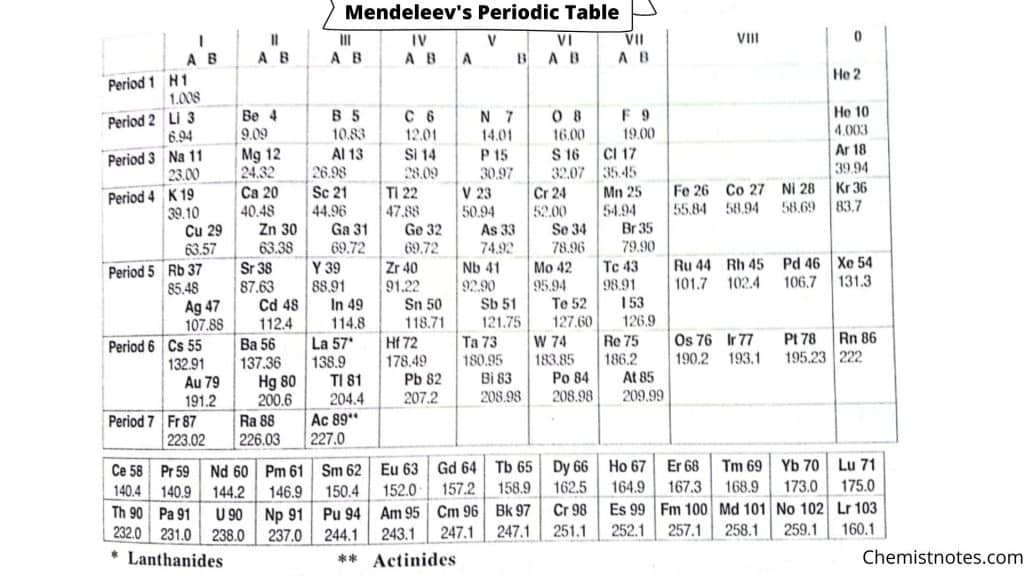
- Nine verticals column called groups: In which the numbered from I to VIII and zero. From group I to VII further divided subgroup as A and B. Group VIII consist of three sets each one containing 3 elements and zero group contains inert gases.
- Seven horizontals rows called periods: In horizontals rows periods are numbered from 1 to 7. In which 1st period is contains 2 elements and 2nd and 3rd periods contains 8 elements and they are called short periods. 4th and 5th periods contains 18 elements and they are long periods. 6th period is longest period in which 32 elements are placed and 7th period is incomplete and contain 19 elements.
Merits of Mendeleev’s Periodic Table
- Mendeleev’s periodic table proved very useful in simplifying the study of elements and their compounds.
- Existence of some undiscovered elements was predicted and Mendeleev left gaps for them.
- Incorrect atomic masses of some of the arranged elements were corrected.
Demerits of Mendeleev’s Periodic Table
- Position of Isotopes: The position of isotopes should be separated according to Mendeleev’s periodic rule but they were kept within the same group.
- Position of Hydrogen: Hydrogen was placed in the group I with alkali metals like Li, Na etc but it could be also placed in the position of halogens.
- Anomalous pairs of elements: Certain elements of higher atomic weights precedes that with lower atomic weights. For example argon (At.wt = 40) precedes with potassium (At.wt = 39) in the periodic table.
- Lanthanides and Actinides: There were no suitable places for Lanthanides and Actinides series.
- Separation of similar but grouping of certain dissimilar elements: Some chemically similar elements like copper and and mercury are placed in different place while some chemically dissimilar elements like copper,gold,silver are placed in same place.
FAQs
Mendeleev’s Periodic Table Was organized by……
Mendelee organized the elements in order of increasing relative atomic weights.
How was Mendeleev’s Periodic Table arranged?
Mendeleev arranged the periodic table in which elements in order of increasing relative atomic mass.
How many elements are there in Mendeleev periodic table?
Mendeleev’s periodic table contains 63 elements.
Problems of Mendeleev’s Periodic Table?
The position of isotopes, Hydrogen was placed in the group I and no suitable places of lanthanides and actinides series.






