Table of Contents
ToggleDisaccharides in a literal meaning mean di refers to two or double and saccharides mean sugar. Simply any compound is composed of two molecules of sugar ( monosaccharide) linked to each other. The most basic type or form of a carbohydrate is a monosaccharide. Be a result, they are referred to as the most fundamental type of carbohydrate. A sugar or carbohydrate that cannot be further hydrolyzed to produce simpler sugars is referred to as a monosaccharide.
Definition of disaccharide
Disaccharides are carbohydrates made up of two molecules of monosaccharide example sucrose, lactose, maltose, etc. They are generally composed of hydrogen, carbon, and oxygen. The general formula of a disaccharide is C12H22O11.
Properties of disaccharides
There are various characteristics of these sugars. They are explained below.
- They are crystalline in nature.
- They are water-soluble.
- They are composed of hydrogen, carbon, and oxygen( hydrates of carbon).
- The chemical formula of these is C12H22O11.
- The covalent bond is present as in other organic compounds.
Classification of disaccharides
They are generally classified into reducing and non-reducing disaccharides.
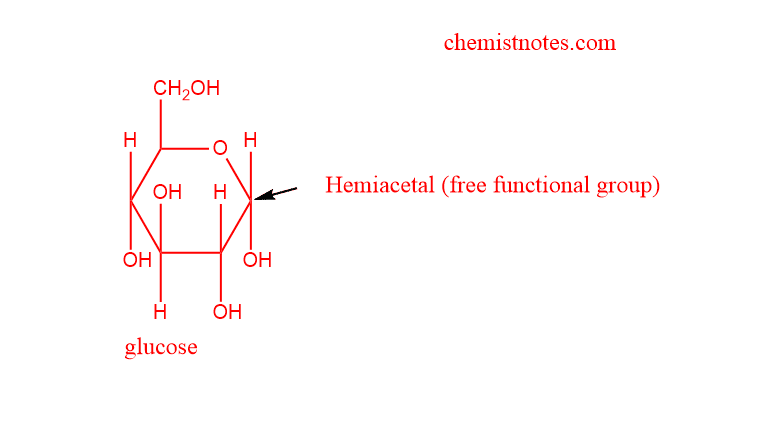
Reducing disaccharide
- Reducing disaccharides are those in which reducing sugar has a free hemiacetal unit that may serve as a reducing aldehyde group.
- Some examples of such disaccharides are maltose and cellobiose.
Non-reducing disaccharide
- Does not act as the reducing agent
- Does not have a free hemiacetal unit.
- examples such as sucrose, and trehalose.
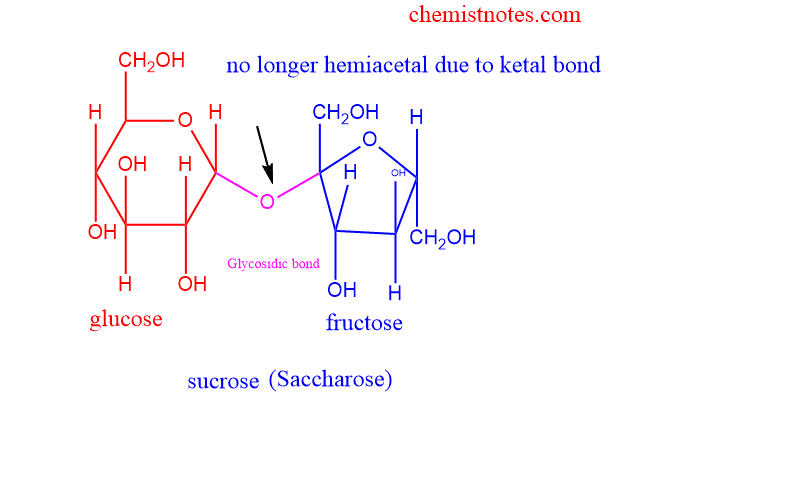
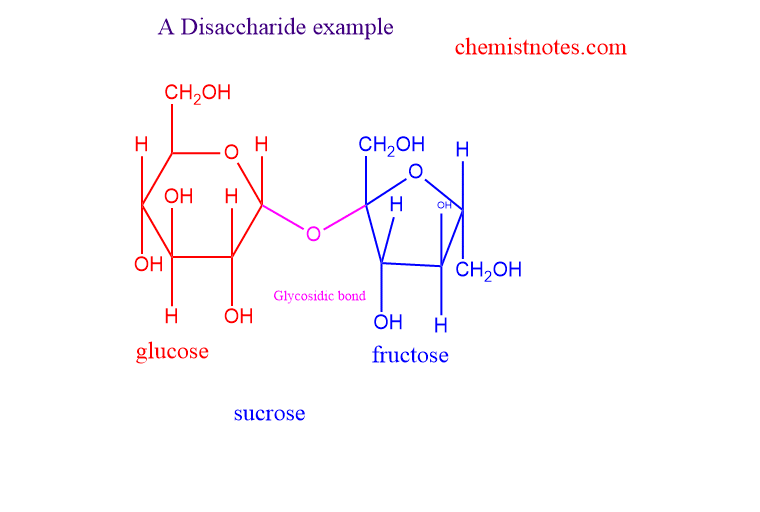
Some examples of disaccharides are explained below:
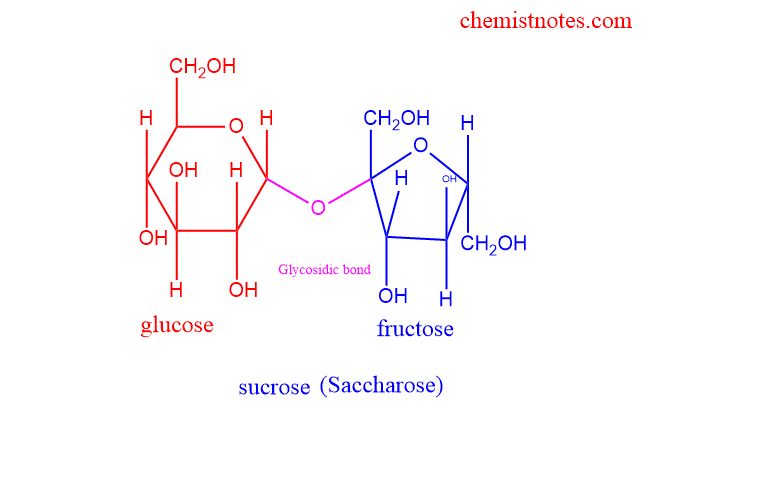
Sucrose
- The common name for sucrose is table sugar or granulated sugar.
- made up of two monosaccharides ie glucose and fructose
- In glucose, the aldehyde group and in fructose, the ketonic group is present
- Linked by the glycosidic bond between c1 and c2.
- Extracted from sugar cane, beetroot, etc
- Generally used to make sweets and baked food, act as sweetening agents
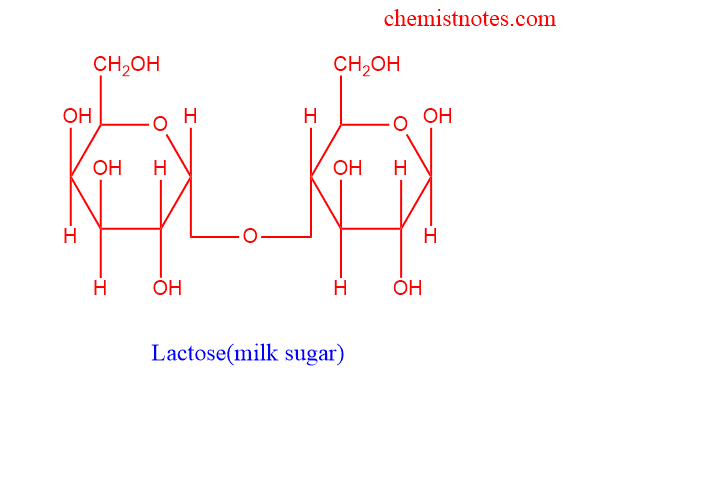
Lactose
- They are also called milk sugar
- They are composed of glucose and galactose
- The chemical formula for lactose is c12h22o11
- Example A cow’s milk has almost 4.7% of lactose
- It is digested by enzymes called lactase
- People who are lactose intolerant cannot digest lactose
- Lactose can be converted into lactic acid
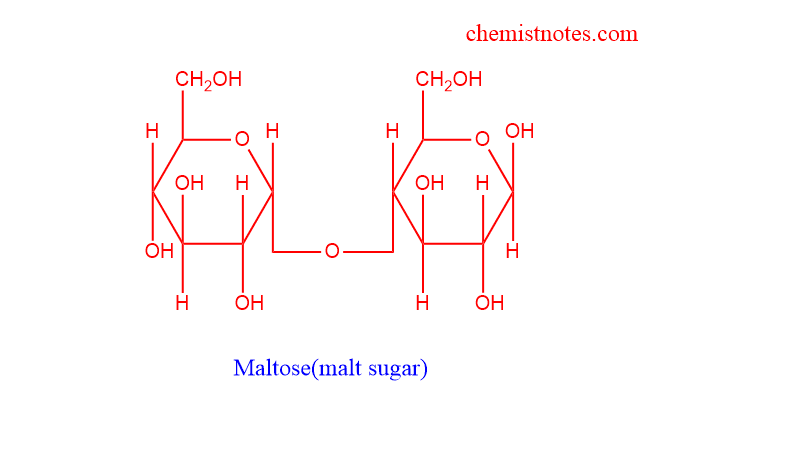
Maltose
- They are also called Malt sugar
- They are composed of two glucose through 1-4 linkage
- They are broken by an enzyme called maltase
- Used as sweetener, culture media, pastries, etc
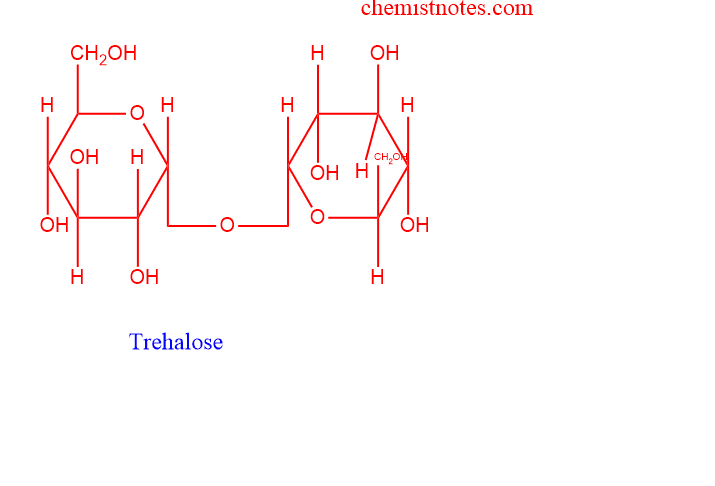
Trehalose
- Trehalose serves as an energy storage chemical in the circulating fluid (hemolymph) of insects, where it is a notable component. The same can be said for yeasts and other parasites.
- In this instance, the two -D-glucose moieties’ two anomeric carbon molecules come into contact with one another.
- As a result, it resembles sucrose in that it is a nonreducing sugar because it does not accumulate free aldehydes. An osazone is not surrounded by trehalose. When hydrolyzed, it produces glucose.
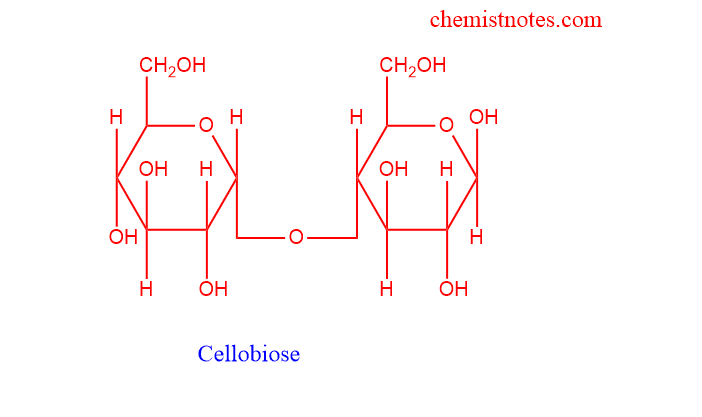
Importance of disaccharides
- They are a good source of energy.
- The human body uses disaccharides as an energy source, and plants also utilize them for a number of purposes (including transporting nutrients around the plant). Since a disaccharide is made up of two monosaccharides, generally known as single sugars, it can also be categorized as a double sugar.
- Chest milk contains lactose, which provides newborns with nutrition. Maltose is a sugar that is frequently included in chocolate and other sweets.
- Sucrose is a carbohydrate that gives the body the energy it needs to carry out both physical and mental tasks. During digestion, the body converts nutrients like sucrose and starch into fructose and glucose. The body breaks down fructose and glucose to give the cell energy.
Osazone formation for dissacharides
Emil Fischer invented osazone formation and used the technique to identify monosaccharides. Both oxidation and condensation reactions are necessary for the synthesis of a pair of hydrazone functionalities. Only “reducing sugars” can function in the reaction since a free carbonyl group is necessary. The nonreducing sugar sucrose does not produce an osazone.
The time for the formation of osazone identifies the types of carbohydrates present in the samples. Every carbohydrate has its different and unique structure of osazone and its time of formation.
Maltosazone, which derives from maltose, crystallizes into petals.
Lactosazone crystals have the shape of powder puffs when made from lactose.
From galactose, galactosazone crystallizes into rhombic-plate-shaped structures.
Broomstick- or needle-shaped crystals of glucosesazone (made from glucose, fructose, or mannose) are produced. Cotton-ball-shaped osazone was produced by lactose.
Differences between sucrose, lactose, and glucose
The most prevalent disaccharide is sucrose, usually referred to as table sugar and composed of the molecules glucose and fructose. Maltose is made up of two glucose molecules, while lactose is a component of milk and is composed of a glucose and galactose molecule. And sucrose falls in the category of non-reducing sugar while lactose and glucose are reducing sugar.
MCQs/FAQs
What are disaccharides and their function?
Disaccharides function similarly to other carbohydrates as a source of energy for the body. When we consume foods containing disaccharides, our systems convert them into monosaccharides, or simple sugars, for absorption in the small intestine.
What is the general formula of disaccharide?
The general formula of a disaccharide is C12H22O11.