Table of Contents
ToggleLaboratory test for protein includes various qualitative test which indicates the presence of proteins. It has certain functional groups which can react to produce characteristically colored products. There are various tests for indicating protein in the given samples. Let us study more about those tests with the easy protocol.
Laboratory test for protein
Protein can be tested by different protocols and methods. First, let us know a few things about proteins. What exactly are proteins? Living things contain chemical molecules called proteins. They perform several different tasks, such as organizing, transportation, and defense. A protein can have up to four different structural levels and is made up of chains of amino acids. Examples of particular proteins are collagen, insulin, and anticorps. Let us talk one by one :
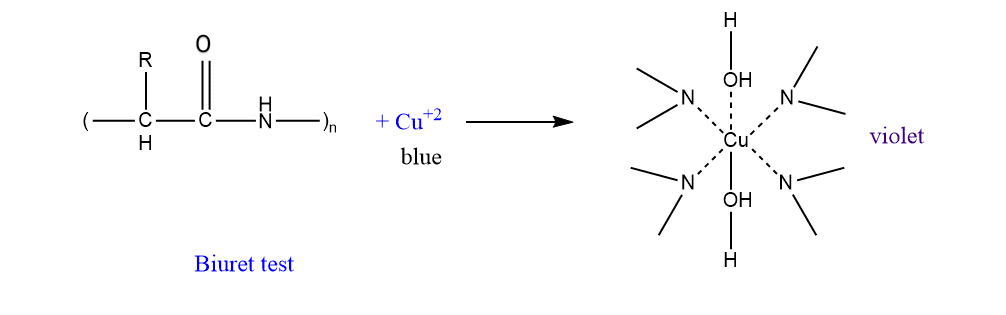
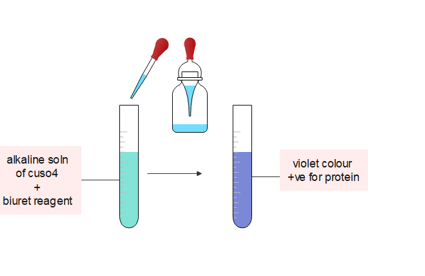
1. Biuret test
This test is specific for peptide bonds present in proteins. The substances containing not less than two peptide linkage give this test. For this test, we have some easy protocols that we can easily carry out in the lab :
Procedure:
- First, the given sample solution is treated with an alkaline solution of dilute copper sulfate and a few drops of biuret reagent is added and observed.
- The presence of violet color indicates the presence of protein in the given samples.
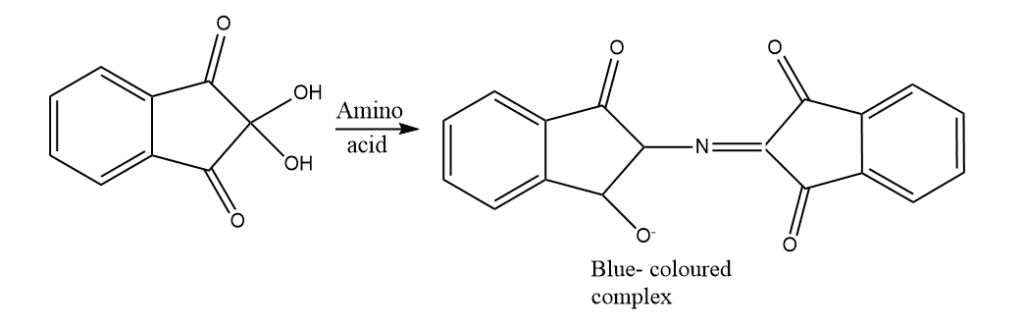
2. Ninhydrin test
A chemical test called the ninhydrin test is used to determine whether an analyte in question contains amines or -amino acids. In this experiment, ninhydrin (a molecule with the formula C9H6O4) was used.
This test results from a reaction between ninhydrin and an amino group of free amino acids. Because of ninhydrin’s potent oxidizing properties, amino acids that are exposed to it undergo oxidative deamination, releasing ammonia, CO2, a matching aldehyde, and a reduced form of ninhydrin ( hydrindantin).
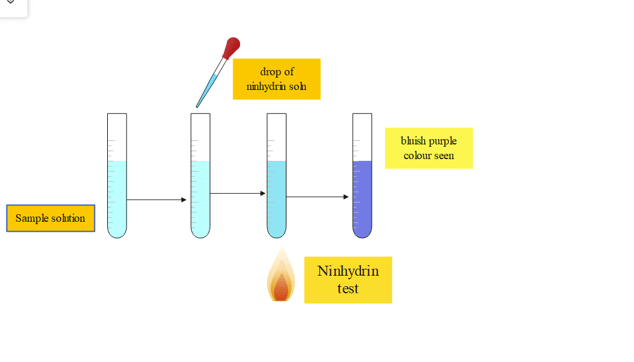
Procedure
| Experiment | Observation | Results |
| Take 1 mL of the test sample in a dried test tube. | ||
| add 10 drops of ninhydrin reagent and hold the test tube on the flame till boiling. | bluish-purple color is seen | Presence of protein |
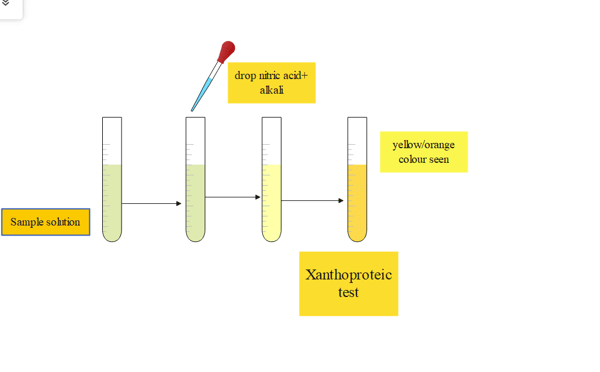
3. Xanthoproteic test
Tyrosine, tryptophan, and phenylalanine are among the amino acids having aromatic nuclei that can be detected using the xanthoproteic test in protein solutions by heating them with concentrated HNO3. Tyrosine or tryptophan will produce a yellow solution when nitric acid is added to a sample and the mixture is heated.
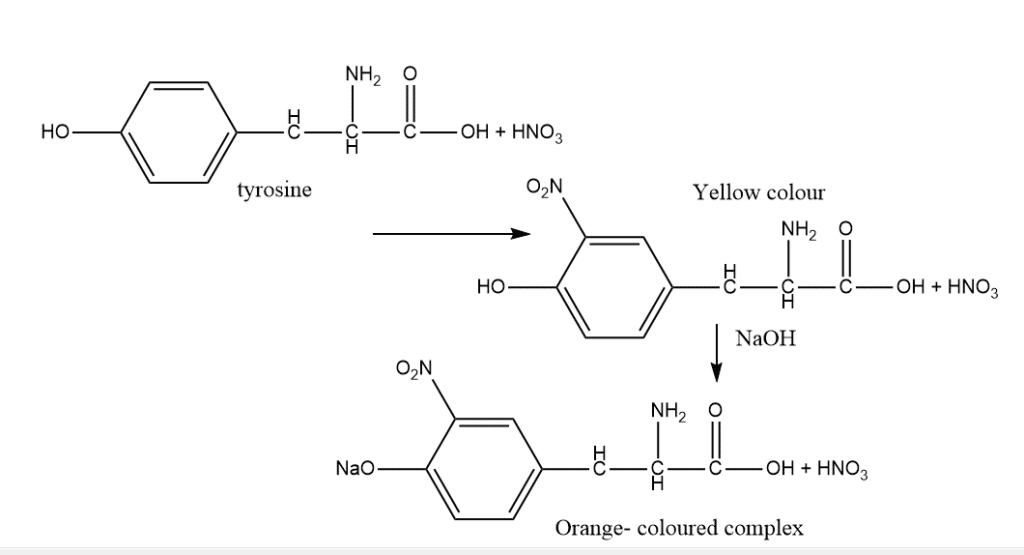
Procedure
| Experiment | Observation | Results |
| Take a clean test tube and a few drops of concentrated sulfuric acid is added and shake the test tube. | ||
| Then gently heat the solution | the appearance of a yellow color | presence of amino acids in the protein |
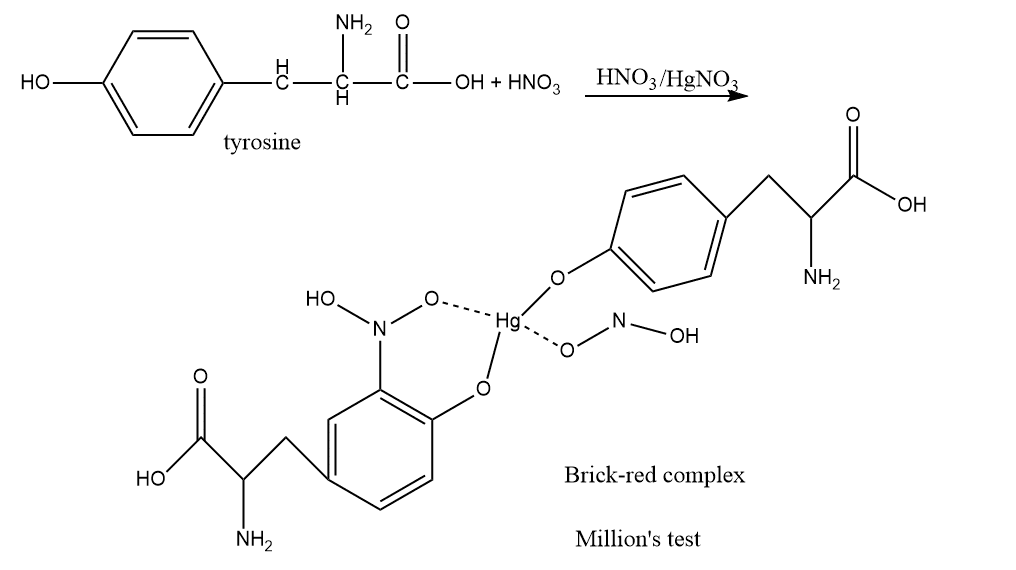
4. Million’s test
Proteins containing phenolic amino acids can give Millon’s test. This test is not provided by gelatin. When proteins are treated with million’s reagents, a white precipitate first forms, and when this precipitate is heated, it turns brick-red, confirming the existence of proteins.
Tyrosine produces a yellow precipitate of mercury-amino acid complex when it reacts with acidified mercuric sulfate solution. The yellow mercury-amino acid combination transforms into the red mercury phenolate upon the addition of sodium nitrate solution and heating.
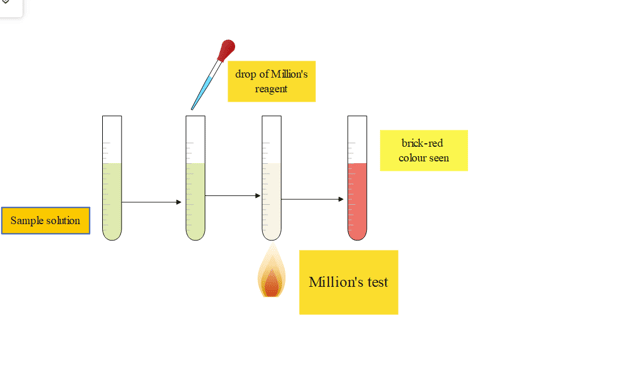
Procedure
| Experiment | Observation | Results |
| 2-3 drops of million’s reagent are taken in a test tube containing the sample | white ppt is seen | |
| then the sample is heated | after heating, it turns into brick red colored | indicates the presence of protein |
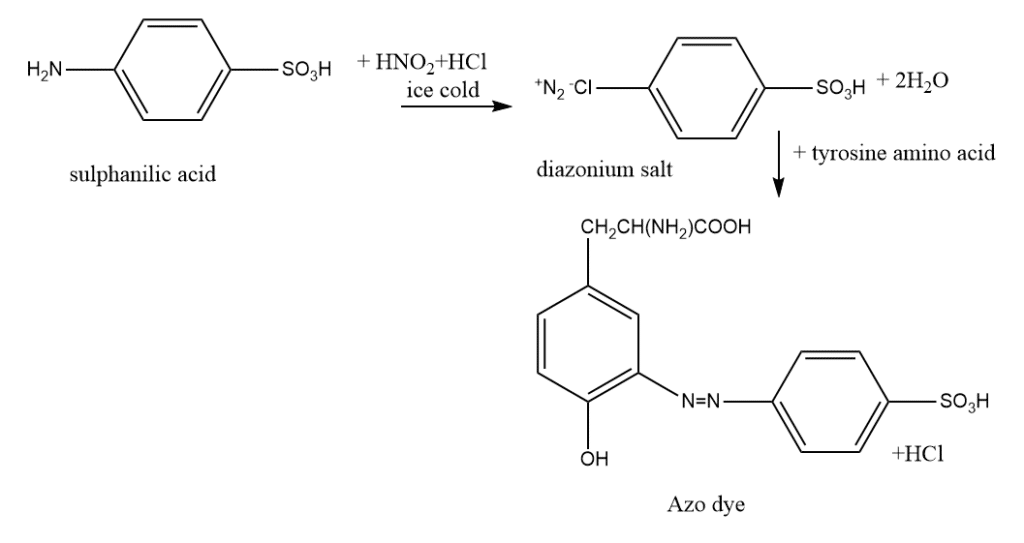
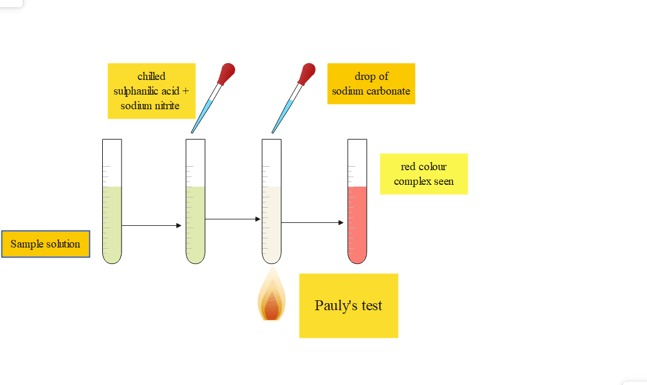
5. Pauly reaction test
This test is specific for the protein containing tyrosine and histidine. Tyrosine or histidine can be found in proteins via the Pauly reaction, a chemical test. Hermann Pauly, a German chemist who first reported the reaction, is honored with its name. When proteins having either tyrosine or histidine react with diazotized sulfanilic acid in an alkaline solution, a coupling reaction results in the formation of red color.
Procedure
| Experiment | Observation | Result |
| Test tube + chilled sulfanilic acid+ few drops of pre-chilled sodium nitrite | color begins to appear | |
| Then 1 mL of sample is added and a few drops of sodium carbonate drop by drop is added | the red colored complex is seen | presence of protein |
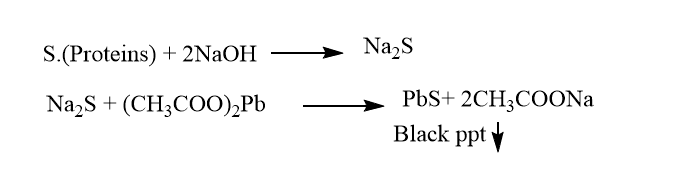
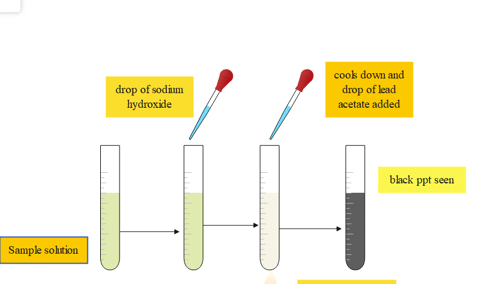
6. Lead sulfide test
This test is used for the specific detection of amino acids like cysteine and cystine. Detection of amino acid containing sulfur, S-S group in cysteine, and S-H group in cystine.
| Experiment | Observation | Result |
| 2 mL of the amino acid solution is taken along with 2mL of sodium hydroxide | ||
| The solution is boiled and cooled down then, a drop of lead acetate is added | the solution turns black (ppt) | presence of cysteine and cystine |
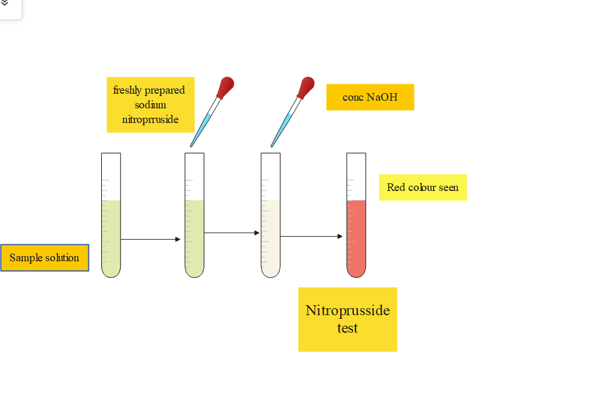
7. Nitroprusside test
This test is especially used to detect the presence of cysteine in the protein sample.
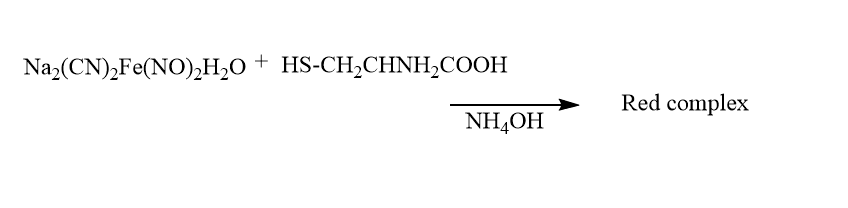
| Experiment | Observation | Result |
| 2mL of amino acid is taken and 0.5 Ml freshly prepared sodium nitroprusside is added | ||
| Now 0.5 concentrated NaOH is added | the red colored complex is observed | presence of cysteine in the given protein sample |