Table of Contents
ToggleSupercritical fluid extraction is one of the advanced solvent extraction techniques used in sample preparation, purification, and concentration of analytes. This technique resembles soxhlet extraction but the main difference is supercritical fluid is used here to extract the target compounds from the mixture. Supercritical fluid extraction plant costs from $85,000 to $300,000. The price may vary with the manufacturer company.
What is supercritical fluid?
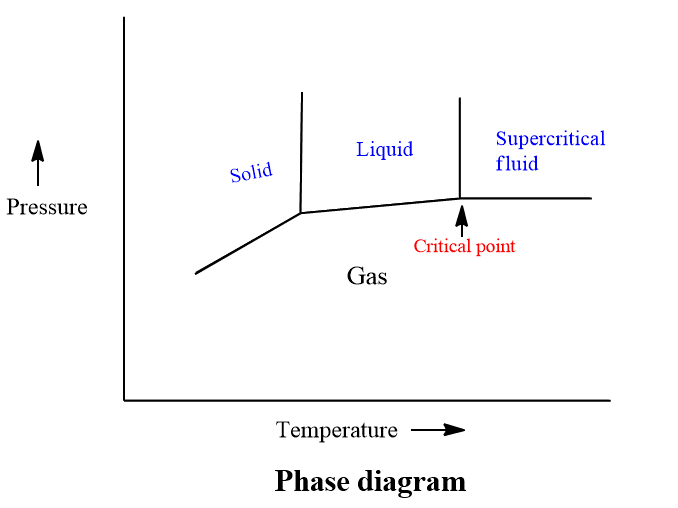
A supercritical fluid is a substance that exists above its critical temperature and pressure and can effuse through like a gas and dissolve materials like a liquid. The properties of a fluid can be changed by changing its pressure and temperature.
Some properties exhibited by supercritical fluids are listed below:
- Liquid-like densities (100-1000 times greater than gases).
- Good solvating power.
- Reduction in surface tension.
- Low viscosity than liquid (10-100) times less than liquid).
- Gaseous-like compressibility.
- Diffusion properties are higher than liquids.
- These show high penetrating power.
Supercritical fluid extraction
Supercritical fluid extraction involves using supercritical fluid as the extracting solvent to separate one component from another (the matrix). This technique is used for the extraction of organic compounds from solid samples.
Carbon dioxide, Ammonia, Ethane, Chlorodifluromethane, Methanol, Nitrous oxide, Water, Xenon, etc. can be used as supercritical fluids or solvents. The most common supercritical fluid in analytical sciences is carbon dioxide.
Advantages of CO2 as supercritical fluid:
- Moderate critical pressure (73.8 bar).
- Low critical temperature (31.1°c).
- High purity at low cost.
- Low toxicity and reactivity
- Ideal for extraction for thermally labile compounds.
- Ideal extractant for non-polar species e.g.alkanes.
- Reasonably good extractant for moderately poor species, e.g. PAHs and PCBs.
- Can directly vent into the atmosphere.
- Little opportunity for chemical changes in the absence of light and air.
- Being a gas at room temperature allows for direct coupling to GC and SFC equipment.
Disadvantages of CO2 as a critical fluid:
The main disadvantage of CO2 is its non-polar nature (it has no permanent dipole moment). The polar substances can be analyzed by the addition of a polar organic solvent or modifier to the supercritical fluid. The addition of the modifier is possible in several ways including the Spiking of organic solvent directly to the sample in the extraction cell, purchase of pre-mixed cylinders, e.g. 10% methanol-modified CO2, or addition of a second pump that allows in-line mixing of CO2 and organic solvent prior to the extraction vessel.
Supercritical fluid extraction principle
This extraction method is based on the critical condition, it refers to the set of temperature and pressure where the liquid and vapor phase of a substance is indistinguishable. The substance beyond this critical point is known as a supercritical fluid.
Since supercritical fluid has a good capacity to dissolve solute, it is thus used to separate solid compounds from the matrix. Some modifiers can be added with solvent to change the polarity and make more selective solvents.
Mainly there are two modes of extraction namely static and dynamic mode. In static mode, the extraction cell containing solute(analyte) is filled with the mobile phase(supercritical fluid) and fluid interacts with solutes. Then, the extracted substance is taken out with the help of a second pump. Whereas, in dynamic mode, the second pump sends the extraction material into the collection chamber continuously during the extraction process.
Supercritical fluid extraction equipment
The major components of a Supercritical fluid extraction system are as follows:
- A supply of high-purity carbon dioxide.
- A supply of high-purity organic modifiers.
- Two pumps
- An oven
- An extraction vessel
- A pressure outlet or resistor
- A suitable collection vessel for quantitative recovery of extracted organic compounds.
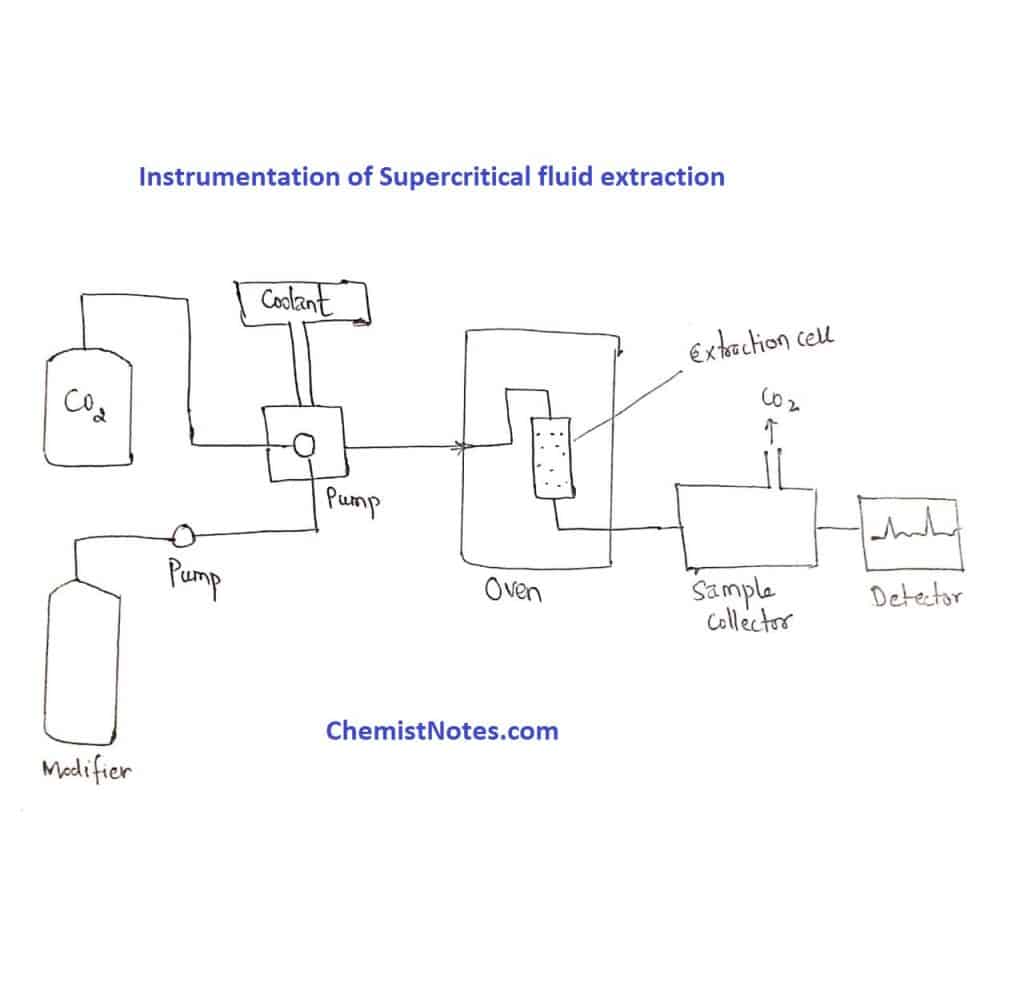
Supercritical fluid extraction instrumentation contains a CO2 cylinder and modifier cylinder (for polar samples). The choice of CO2 is an important initial consideration as far as impurities are concerned. It is essential that the level of impurities encountered in the CO2 doesn’t interfere. With subsequent analysis, the CO2 is supplied in a cylinder fitted with a dip tube which allows a syringe pump. (Note: It is possible to purchase cylinders that contain both CO2 and organic modifier, e.g.10% methanol-modified CO2).
.To allow pumping of the liquefied CO2, without cavitation, requires the pump head to be cooled. This is achieved by using a jacketed pump head which is either cooled via an ethylene glycol mixture pumped using a recirculating water bath or a ‘ peltifier’ device. If the modifier is to be added via a second pump, the CO2 and modifier are mixed using T- a piece. Liquefied CO2 is pumped by a reciprocating or syringe pump.
To achieve this, the required critical temperature requires the extraction vessel containing the sample to be located in an oven that is capable of effective controlled heating in the range of 30-250°c. The sample vessel, made of stainless steel, must be capable of withstanding high pressures (up to 10000 psi) safely. The sample is located inside the extraction vessel. Pressure is established by using a variable restrictor.
The sample is placed in an extraction vessel and pressurized with SCF CO2 to dissolve the sample. After extraction, the extract is transferred to the fraction chamber and depressurized due to which CO2 loses its solvating power causing the entire material to precipitate. Now the CO2 gas is recycled. The precipitated material is extracted with the addition of a small amount of solvents.
Advantages of supercritical fluid extraction
- Easy elimination of organic solvents i.e. eco-friendly.
- It is fast due to the quick back diffusion of analytes in the SCF reduces the extraction time since the complete extraction step is performed in about 20 min.
- It is suitable for the extraction and purification of compounds having low volatility present in solid or liquid.
- Susceptible to thermal degradation ( low operating conditions).
Applications of supercritical fluid extraction
- It is used for the extraction of various natural products. E.g. coffee, tea, spices, etc.
- It is used to extract essential oils from black pepper.
- Quercetin from onion skins, Epicatechin from sea coat of sweet Thai, and Tamarind can be extracted.
- It is used to determine the fat content of numerous products from beef to oil seeds and vegetables. E.g. fat content in soybean, sunflower, cottonseed, etc. It has higher recoveries.
- SFE is used to determine the pesticides in foods.






