Table of Contents
ToggleSeparation of one kind of chemical species from other is one of the most important steps in chemical analysis. Among several separation techniques, solvent extraction, also known as liquid-liquid extraction is the most versatile, popular, and widely used technique. This technique can be used for the preparation, purification, and separation of chemical species.
Solvent extraction
Solvent extraction is one of the separation methods in which one component or solute present in one phase is extracted into another phase by using a suitable solvent. Thus, the process by which a solute is transformed from one phase to a new phase is known as extraction.
This process of extraction using a suitable solvent is popularly known as solvent extraction or liquid-liquid extraction. The common extraction solvents used are ether, chloroform, benzene, CCl4, ethyl acetate, etc.
In simple liquid-liquid extraction, the solute is partitioned between two immiscible phases(solvents).In most cases one of the phases is aqueous and the other phase is an organic solvent such as ether, or chloroform. Because the phases are immiscible, they form two layers with the denser phase on the bottom. The solute is initially present in one phase but after extraction, it is present in both phases.
Principle of solvent extraction
Solvent extraction is also called liquid-liquid extraction. It is based on the fact that a particular solute distributes itself in a fixed ratio between two immiscible solvents.
A target solute may be soluble in two different solvents but the extent of solubility may be different, provided that solvents are immiscible with each other. When solutions of a particular solute in two immiscible solvents (say aqueous and organic solvents) are mixed, the solute distributes itself from one phase to another phase in a fixed ratio. This is the underlying principle of solvent extraction.
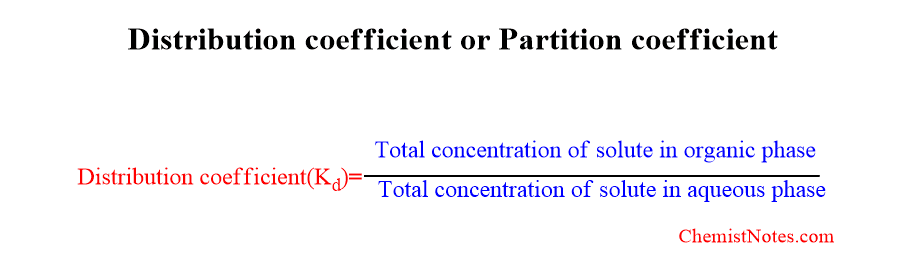
The Nernst distribution law can be applied in solvent extraction. According to this law, the distribution coefficient or partition coefficient Kd is fixed for a solute in pair of immiscible solvents.
Solvent extraction equipment
Liquid-liquid extraction is usually performed with a separatory funnel, which is one of the widely used solvent extraction equipment in the laboratory.
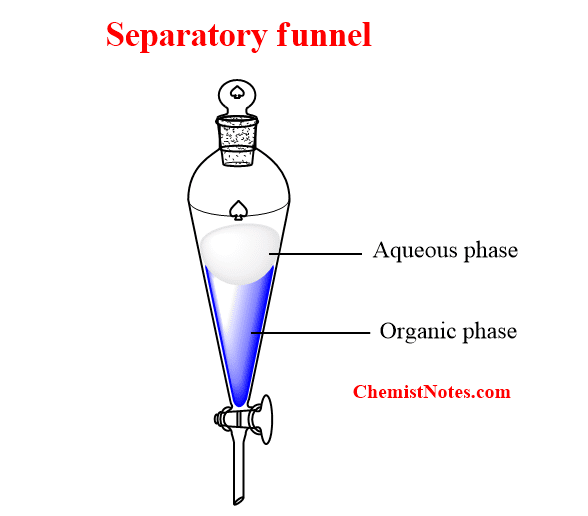
In solvent extraction, either a solute is extracted from an aqueous solution into an immiscible organic phase, or a solute is extracted from the organic phase into an aqueous solution. During extraction, a solute to be extracted from a solution either aqueous or organic is taken in a separating funnel and another solvent in which the solute is desired to be extracted (organic or aqueous) is mixed. Then, the mixture is thoroughly shaken for a definite period. Then after, it is allowed to settle down into two layers. The denser layer is the bottom layer and it is drawn off.
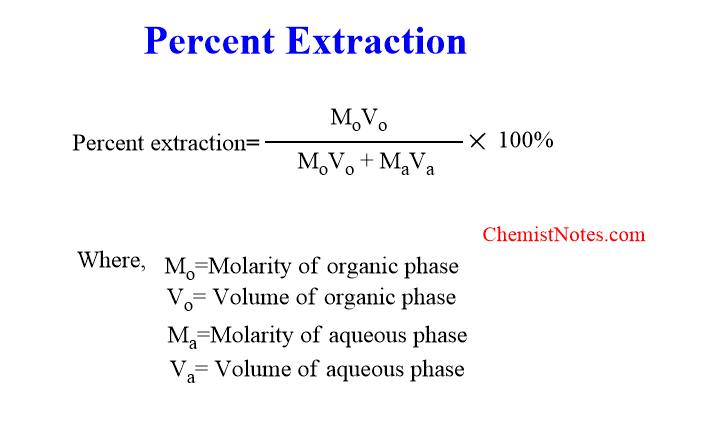
Percentage extraction in solvent extraction
It is possible to determine the amount of solute extracted from the solution when solute molecules do not undergo association, dissociation, or combination with solvent.
Thus, percentage extraction can be defined as the amount of solute extracted out of per hundred parts of solute distributed in a pair of immiscible solvents. Mathematically, it can be expressed as shown below:
liquid-liquid extraction solvent selection
If removal of dissolved substance present in aqueous solution by using another solvent, usually organic solvent such as ether, chloroform, benzene, carbon tetrachloride, is done. These organic solvents are called extracting solvents.
The characteristics of a good extraction solvent are stated below:
- Extracting solvents should be completely immiscible with the original solvent.
- The solute to be extracted must have greater solubility in the extracting solvents
- In order to remove the solute by heating the solution in a water bath, the extracting solvents must be more volatile than the solute.
Application of solvent extraction
It is one of the most important separation techniques used in environmental, clinical, and industrial laboratories. Few applications have been mentioned here.
- Public drinking water supplies are routinely monitored for trihalomethane, which is known for carcinogenicity. These are extracted from their aqueous matrix by liquid-liquid extraction using pentane as extracting solvent.
- In Parke’s process for desilverization of lead, this technique is used.
- Orange juice is also tested for the presence of organophosphorus pesticides using liquid-liquid extraction. Acetonitrile is combined with a sample of orange juice before filtering. Petroleum ether is used to remove any potential organophosphorus pesticides from the filtrate.
Solvent extraction video
FAQs/MCQs:
what is distribution coefficient in solvent extraction?
Distribution coefficient can be defined as the ratio of total concentration of solute in organic phase to that of solute in aqueous phase. It is also called partition coefficient. It is denoted by Kd.
References:
- S. M. Khopkar, Basic Concepts of Analytical Chemistry, (3rd Edition), New Age International Pvt. Ltd., New Delhi, India (2008).






