Table of Contents
ToggleSolid phase extraction(SPE) was developed from classical chromatography in which an adsorbing medium is used to separate analytes according to their differing equilibrium affinities for the sorbing medium. This technique is also called liquid-solid extraction.
What is solid phase extraction?
Solid phase extraction(SPE) is one of the analytical techniques for the sample preparation that concentrates and purifies analytes from solution by sorption onto a solid phase, followed by elution of the analytes with a solvent.
In the traditional method, sample preparation involves sample dissolution, purification, and extraction that was carried out with liquid-liquid extraction or solvent extraction. The disadvantages of liquid-liquid extraction include:
- Use of large volumes of organic solvents.
- it creates emulsions with aqueous samples which are difficult to extract.
- Liquid-liquid extraction is not easily automated.
- It involves cumbersome glassware and cost.
To overcome these mentioned difficulties, solid-phase extraction was invented in the mid-1970s as an alternative approach to liquid-liquid extraction.
What is the goal of solid phase extraction? Some of the main goals are listed below:
- To remove analyte quantitatively from solution and completely recovers it in an appropriate solvent.
- Purification of analytes involves the removal of analytes from interfering compounds.
- Concentrating the analytes in a small volume of solvent.
Multimodal SPE
SPE normally takes place using one device with a single sorbent. However, if more than one type class of compound is present in the aqueous sample, then multimodal SPE can be used. Multimodal SPE can be done in one of the two ways:
- Either by connecting two alternate phase SPE cartridges in series.
- By having two different functional group sorbents present within one cartridge.
Solid phase extraction principle
Solid phase extraction normally involves bringing an aqueous sample into contact with a solid phase or sorbent, whereby the compound is selectively adsorbed onto the surface of the solid phase. The solid phase sorbent is usually packed into small tubes or cartridges. By careful selection of the sorbent, the organic compounds should be retained by the sorbent in preference to other extraneous materials present in the sample. This extraneous material can be washed from the sorbent by the passing of an appropriate solvent. Subsequently, the compounds of interest can then be eluted from the sorbent using a suitable solvent. This solvent is then collected for analysis.
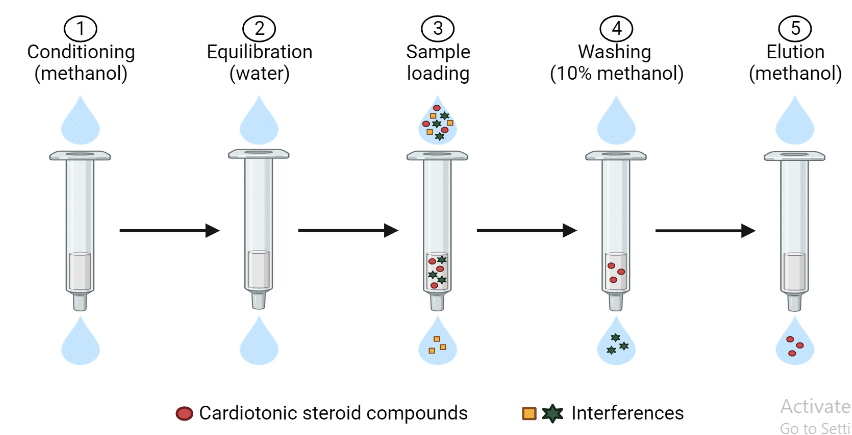
Solid phase extraction procedure
The process of solid phase extraction completes in four steps:
- Conditioning of sorbent
- Loading step
- Washing of sorbent
- Elution
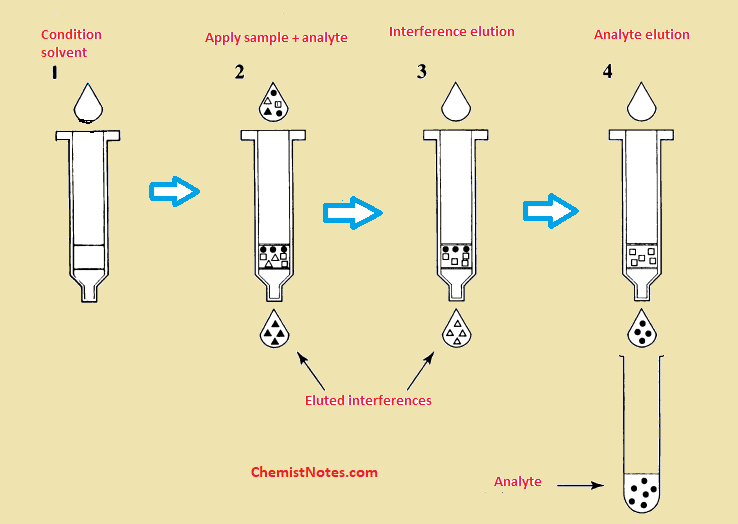
Conditioning of sorbent
In this step, a solvent is passed through the sorbent to wet the packing materials and solvate the functional groups of the sorbent. During this process, the air present in the column is removed and void spaces are filled with solvent.
A commonly used conditioning solvent is methanol which is then followed with water or an aqueous buffer. If the sorbent dries for more than several minutes under a vacuum, the sorbent must be reconditioned. Otherwise, the mechanism of sorption will not work effectively and recovery of the analyte will be poor.
Loading of sample
In this step, the sample and analyte are applied to the column. The mechanism of retention holds the analytes on the column while the sample is added. The mechanisms of retention include Van der Waals interaction, hydrogen bonding, dipole-dipole forces, size exclusion, and cation and anion exchange.
During this step, analytes are concentrated on the sorbent. Similarly, some of the matrix compounds may also be retained.
Washing of sorbent
In this step, a suitable solvent is used to remove the sample matrix and other impurities from the interstitial spaces of the column while retaining the analytes. If the sample matrix is aqueous, an aqueous buffer or a water-organic solvent may be used.
Elution of analytes
In this step, an appropriate solvent is used to disrupt the analyte-sorbent interaction so that analyte is eluted from the sorbent column. The eluting solvent should remove as little as possible of the other substance sorbed on the column.
Solid phase extraction equipment
There are three types of column or apparatus used in Solid phase extraction. These are disks, cartridges, and syringe barrels. Among them, the syringe barrel is the most commonly used form for SPE. It consists of 20 µm polypropylene a the bottom with 40 µm-sorbent materials and 20 µm-polypropylene frits at the top.
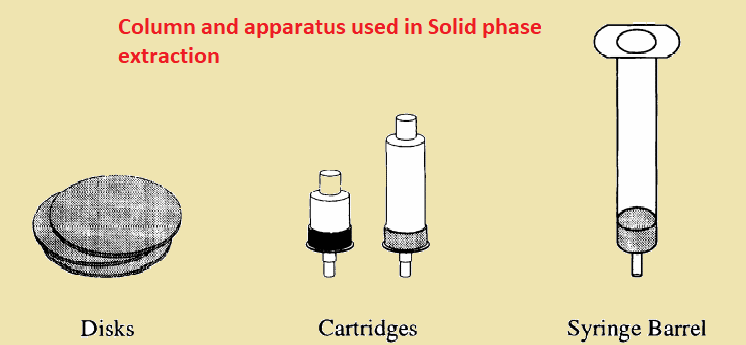
solid phase extraction cartridge
It can be further classified as single and multiple cartridges. A brief description of these is given below:
- Single cartridge: The most common arrangement is the syringe or cartridge, usually made from polypropylene (other types: glass and polytetrafluoroethylene, PTFE, are also available) with a wide entrance, through which the sample is introduced, and a narrow exist. The appropriate sorbent material (50 mg to 10 g) is put at the base (exist) of the cartridge. Solvent flow through a single cartridge is typically done using a side-arm flask apparatus.
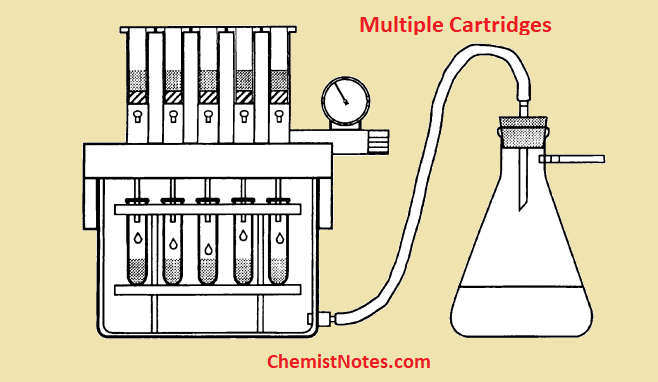
- Multiple cartridges: Multiple cartridges can be simultaneously processed ( from 8 to 30 cartridges). In both cases, a vacuum pump is required for the movement of solvent/ sample through the sorbent.
Types of sorbent and modes of interaction
Mainly there are three types of sorbent used in solid phase extraction namely:
- Normal phase sorbent
- Reverse phase sorbent
- Ion exchange sorbent
Normal phase sorbent
- It consists of a stationary phase that is more polar than the solvent or sample matrix.
- When the sample is an organic solvent containing the analyte, normal phase sorbents are used.
- Polar interactions such as hydrogen bonding, and dipole-dipole interactions are the primary mechanism of solute retention.
- Sorbents have the polar functional group. The polar nature of these sorbents means that it is more likely to retain polar compounds.
- Example: Alumina, silica gel, cyano, amino, diol, etc.
Reverse phase sorbent
- These are packing materials, which are more hydrophobic than the sample.
- These sorbents are commonly used when aqueous samples are involved.
- The mechanism of interaction is van der Waal’s forces and occasionally secondary interactions such as H-bonding, and dipole-dipole interactions.
- Sorbents have the non-polar functional group and these are more likely to retain non-polar compounds.
- Example: Octadecyl(C-18), Octyl(C-8), Ethyl(C-2), Graphitized carbon etc.
Ion exchange sorbent
- Sorbents have either cationic or anionic functional groups.
- When these functional groups are in ionized form, attract compounds of the opposite charge.
- A cation exchange phase, such as benzene sulfonic acid, will extract a compound with a positive charge.
- Similarly, an anion exchange phase will extract a compound with a negative charge.
- Example: Quatnery amine, carboxylic acid, aromatic sulfonic acid, etc.
Factors affecting SPE
- Nature of SPE sorbent: The choice of SPE sorbent is highly dependent upon the compound of interest and the sorbent system to be used.
- Choice of solvent: It depends on the nature of the analytes.
- Flow rate of sample: The flow rate of the sample through the sorbent is important; too fast in flow will allow minimum time for compound sorbent interaction. This must be carefully balanced against the need to pass the entire sample through the cartridge or disc. It is normal therefore for an SPE cartridge to operate with a flow rate of 3-10mL min-1 whereas 10-100 mL min-1 is typical for the disc format.
Advantages of solid phase extraction
- The amount of solvent required for the clean-up is greatly reduced.
- Saving time for evaporative concentration.
- Minimizing exposure of analysts to the toxic solvent.
- The final elute has less interfering material.
- Accuracy and precision are improved.
- The sample prepared by SPE can easily be analyzed by different techniques. e.g. HPLC
- It is simple and safe to use.
- Ease of automation.
- High recoveries of analytes or purified extracts.
Application of solid phase extraction
Due to its flexibility, it is applicable in environmental, clinical, and pharmaceutical sample preparation and purification. For example:
- The concentration of trace organic pollutants from water.
- Purification of drugs of abuse from blood and urine.
- Extraction of organic compounds from food and beverages.
- Impurity profiling of pharmaceutical.
- Applications to biological fluids.






