Table of Contents
ToggleThe electrode potential and emf of a cell depend on the nature of electrode temperature and the activities of the ions in the solution. The variation of electrode and cell potential with the activities of the ions in solutions can be given by the Nernst equation. This equation, formulated by Walter Nernst in 1889, can be used to calculate the half-cell potential.
What is the Nernst equation?
Nernst equation is a mathematical expression that shows the effect of the concentration of the solution on the electrode potential of the cell. This equation can be used to determine either the individual electrode potential or the cell potential under non-standard conditions.
Nernst equation derivation
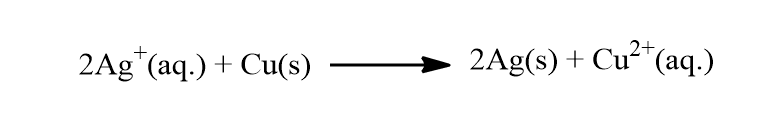
The Nernst equation can be derived by Van’t Hoff reaction isotherm. Let’s consider a reaction as shown below:
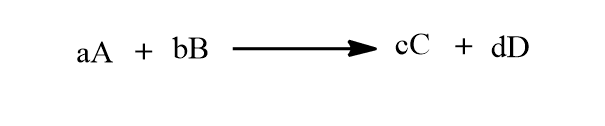
The free energy change of the reaction in the non-standard conditions at constant temperature is given by,
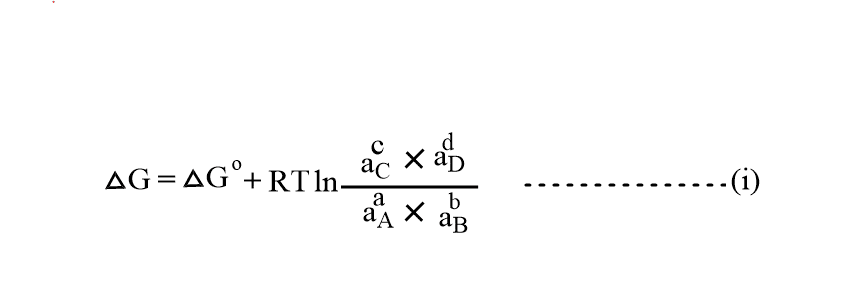
This equation is known as Van’t Hoff reaction isotherm.
Where, 𐤃G= free energy change of reaction in non-standard condition
𐤃Go= free energy change of reaction in standard condition.
aA and aB= activities of the reactants.
ac and aD= activities of products.
Now, the work done by the galvanic cells is equal to the decrease in the free energy change of the system. So,
-𐤃G= nFE cell and -𐤃Go= nFEocell
Substituting these values in the above equation(i), we get;
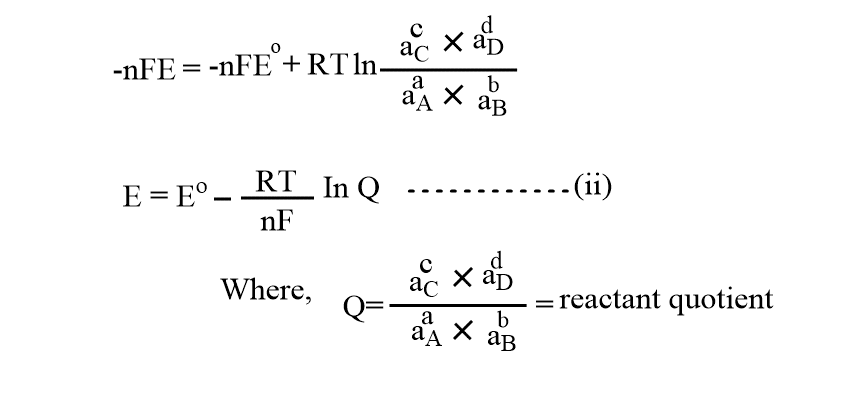
Equation(ii) is known as the Nernst equation. There are two cases:
Case 1: If Nernst’s equation is applied to a single electrode reaction( half cell reduction reaction), it will be in the form given below:
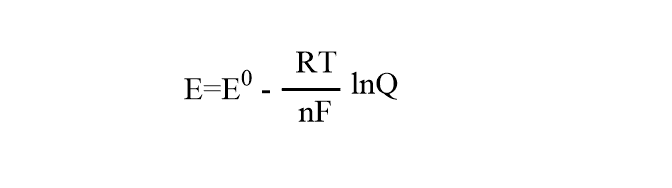
E= Electrode potential under non-standard condition
Eo=electrode potential under standard conditions.
R= gas constant(8.314 Jmol-1 k-1)
T= temperature in Kelvin
n= electron change in moles
F= faraday’s constant(96500 C)
Q= reaction quotient for electrode reaction.
Case 2: If Nernst’s equation is applied to the complete cell as a whole then it will be in the form:
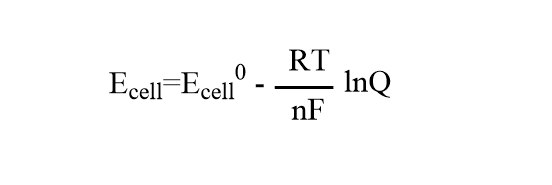
Ecell= Emf of a cell under non-standard conditions.
Eocell= Emf of the cell under standard conditions
Q= reaction quotient for the cell reaction.
Applications of Nernst equation
- The Nernst equation can be used to calculate the electrode potential of any electrode at any condition.
- Emf of the cell can be determined.
- The concentration of the unknown ionic solution can be determined.
- PH of the given solution can be calculated.
- The solubility of sparingly soluble salts can also be calculated using this equation.
Limitations of Nernst equation
This equation can not be used to measure cell potential when the current flows through the electrodes since it changes the activity of ions present on the electrode surface.
Numerical problem
What is the potential of a copper-silver cell of Ag+ concentration is 1.0 x 10-3 M and Cu2+ id 1.0 x 10-4 M. The standard potential of the cell is 0.46 V and the cell reaction is given as: