Table of Contents
ToggleThe water content or percent humidity present in numerous manufactured products, including butter, cheese, dried milk, sugar, various solvents, and other industrial products like paper, gas, petroleum, and plastic films, can be determined. There are some methods available to do so, but Karl Fischer titration is a widely used method for the determination of water content in the sample.
Definition of Karl Fischer titration
Karl Fischer titration can be defined as an analytical technique that uses coulometric or volumetric titration to determine a trace amount of water in a sample quantitatively.
Principle of Karl Fischer Titration
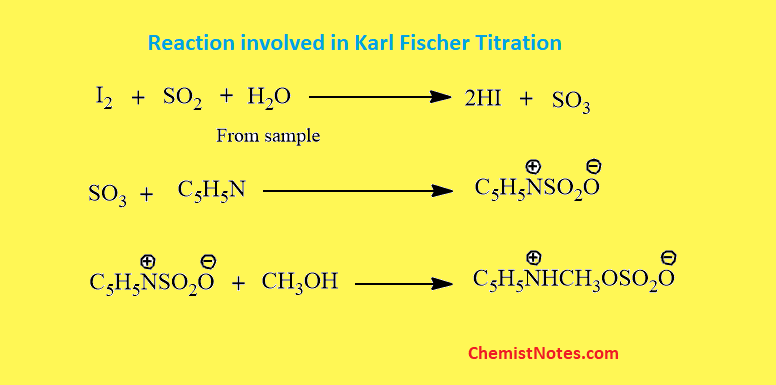
The principle of Karl Fischer titration is based on the redox reaction between iodine and sulfur dioxide in presence of water. Water reacts with iodine and sulfur dioxide to form sulfur trioxide and hydrogen iodide. An endpoint is reached when all the water present in the sample is consumed. The endpoint is detected by the sudden drop of a current during the endpoint. A constant current is detected during titration but at the endpoint, there is a sudden drop in current.
The reaction uses a non-aqueous system containing excess sulfur dioxide with primary alcohol usually methanol as a solvent which serves as both a diluent and a reaction medium. Pyridine is also used as a base and a buffering agent.
The chemical reaction involved during Karl Fischer’s titration is shown below:
Problem of Karl Fischer titration
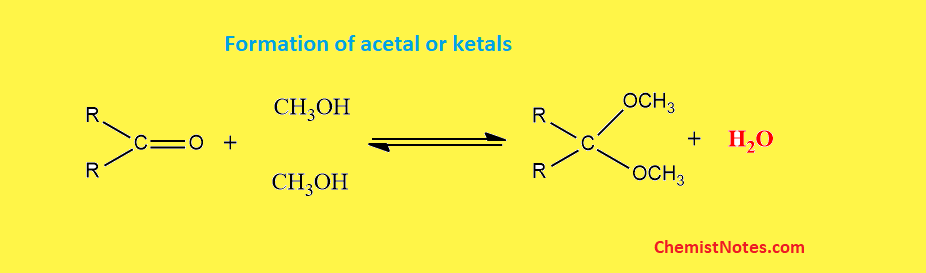
One of the major problems of this titration is that aldehydes and ketones can not be analyzed by this method because these react with methanol to form acetals and ketals respectively, with concomitant production of water. This produced water is also titrated, hence resulting in the wrong result of water content.
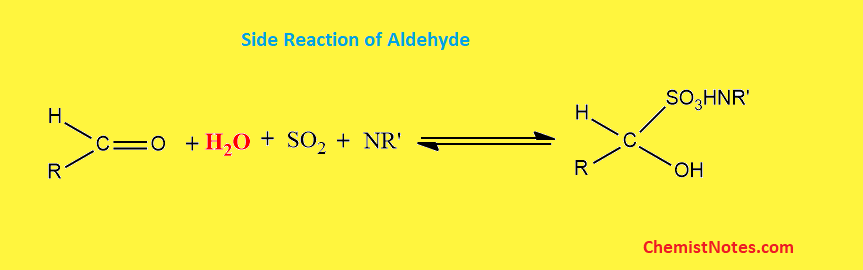
Similarly, aldehyde undergoes a side reaction with bisulfite, which consumes water during the reaction. Therefore, this kind of reaction leads to incorrectly low water content.
Karl Fischer titration reagent
For volumetric Karl Fischer titration, there are mainly two types of reagent.
- One component KF reagent: Iodine, sulfur dioxide, and imidazole are all present in the titrant and are dissolved in appropriate alcohol. Methanol serves as a solvent. With the one-component reagent, titration speed is slower. But this regent is easy to handle.
- Two-component KF reagent: Iodine and methanol are components of the titrant. Sulfur dioxide, imidazole, and methanol are present in the solvent. With the two-component reagent, titration can be done at a speed that is two or three times higher.
Types of Karl Fischer titration
The water content determination by this method can be performed nowadays by two different techniques.
- Volumetric Karl Fischer titration
- Coulometric Karl Fischer titration
The selection of the appropriate technique is based on the estimated water content in the sample.
Volumetric karl fischer titration
It is suitable for samples with high water content. The water is measured in mg. Thus, this method is not suitable for the sample if a trace amount of water is present. At least 10 mg of water must be present in the sample for the volumetric technique to produce results with an acceptable level of precision.
In this titration, a solution containing iodine is added directly from the burette during titration.
Coulometric karl fischer titration
The coulometric Karl Fischer titration has many benefits when the water concentration is as low as 10 μg. Coulometric Karl Fischer titration has the same chemistry but the main difference is the way of introducing iodine into the titration cell.
The iodine required for the reaction is generated from a precursor by applying an electrical pulse to the electrode. The KF reagent contains an iodine precursor which is oxidized to iodine in contact with the working anode.
Application of Karl Fischer titration
- It helps to increase product stability. e.g: Pharma products or food products.
- It is applicable to stop the growth of microorganisms by determining water content.
- An analytical technique for quantitative analysis of total water content.
- It is used in the food industry for water content determination in fruit juice, honey, flour, noodles, chips, and cocoa powder.
- It is used in the petroleum industry to determine water in different oils, gasoline, kerosene, and petroleum.
- It is used in cosmetic industries for the determination of water in shampoos, creams, lipstick, and toothpaste.
- It is used in pharmaceuticals for raw materials, active substances, tablets, ointments, oils, etc.
- It is useful in the determination of water in silk, wool, wood, paper, and even in building materials such as zeolites and cement.
Advantages of Karl Fischer titrations
- High accuracy and precision
- Selectivity for water
- Short analysis duration
- Easy sample preparation
- Suitable for analyzing solid, liquid, and gases.
Limitations of Karl Fischer titrations
- Inferences of the compound with iodine.
- Highly acidic or basic compounds can not be analyzed.
- A large amount of samples is needed in some cases.
- water in the carbonyl group is difficult to determine.






