Table of Contents
ToggleStructure of zeolite, a general introduction of zeolite, synthesis, properties, and application have been discussed briefly in this post.
Zeolites are crystalline solid structures made up of silicon, aluminum, and oxygen that form frameworks with cavities and channels inside where cations such as K+, Na+, Ca2+, water, or small molecules may reside. The general formula of zeolite is Mn+ x/n [Alx Siy O2x+2y]2+. ZH2O, where M is metal of valency n.
Many zeolites occur naturally as minerals but most of these have been made synthetically. These have wide applications in petrochemical cracking, water softening, water purification, separation and removal of gases and solvents, medicine, industry, etc.
Most of us may get confused between Molecular sieve and zeolites. Actually, the molecular sieves are porous solids with pores of the sizes of molecular dimensions 0.3-2.0nm in diameter. Zeolite is an example of a molecular sieve.
Structure of Zeolite
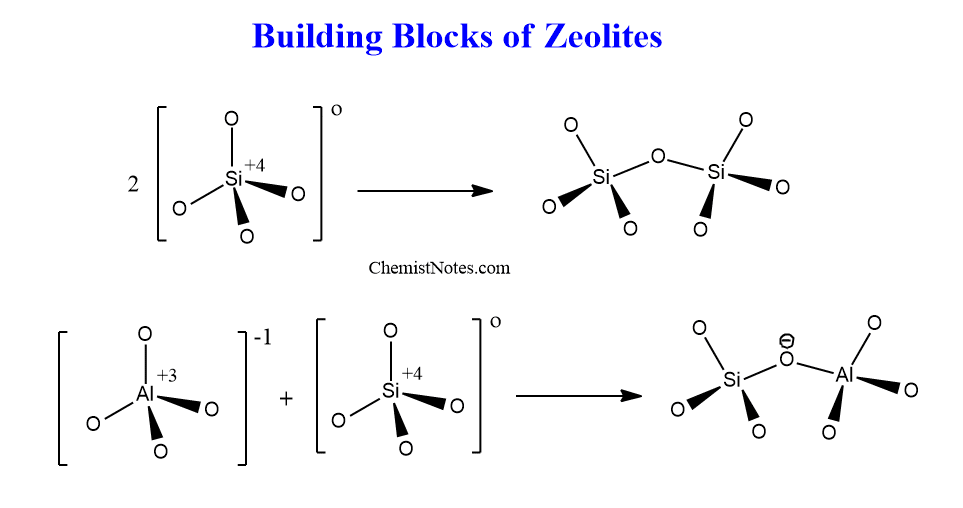
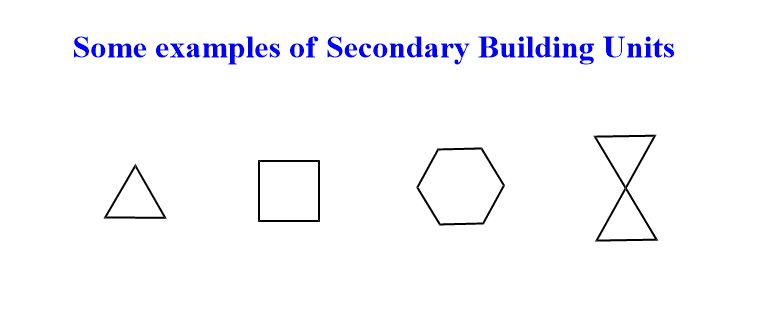
The structure of zeolites can be categorized into primary building units(PBUs) and secondary building units(SBUs). Primary building units are (SiO4)4-, (AlO4)5- tetrahedral units. These tetrahedral primary building units combine with adjacent tetrahedral by sharing oxygens atoms to form a spatial arrangement of simple geometric forms called secondary building units. The secondary building units have different forms such as single rings, double rings, polyhedral, or even more complex units such as channels and cages.
Zeolite consists of three-dimensional tetrahedral networks of silicon and aluminum which are linked by common oxygen atoms. Therefore, pores are formed, hence zeolites are a group of microporous materials having pore sizes less than 2 nm.
Chemical composition of zeolites
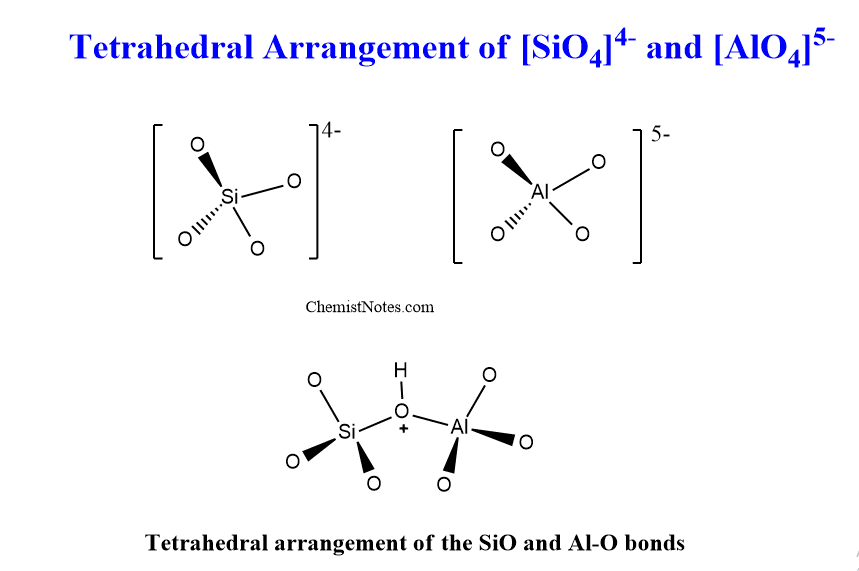
All zeolites are composed of a tetrahedral arrangement of silicon cations (Si4+) and aluminum cations (Al3+). These are surrounded by four anions O2- forming tetrahedral.
Synthesis of zeolites
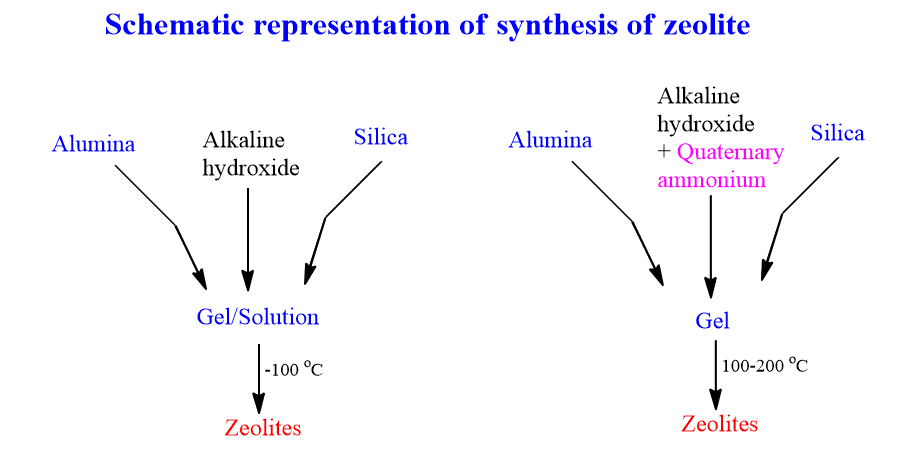
Zeolites are synthesized from a gel or solution containing silica and alumina at high pH in an alkaline solution. The first step involves the dissolution of aluminum and silicon to form aluminate and silicate anions. These are then brought together to form a gel by condensation/polymerization.
Properties of Zeolites
Some important properties of zeolites are listed below:
- Zeolites are very stable solids having a high melting point of about 1000 degrees Celsius.
- They are insoluble in water and other inorganic solvents
- They don’t undergo oxidation in the presence of air.
- The characteristic property of zeolites is their open-cage-like framework which helps zeolites to trap water and ions such as Na+, K+, Ca++.
- Naturally found zeolites do have not uniform pore sizes but synthetic zeolites are synthesized in a very precise manner with uniform pore sizes.
- Zeolites rich in alumina are attracted to polar molecules like water but zeolites rich in silica are attracted to non-polar molecules.
Applications of Zeolites
Some major applications of zeolites are listed as:
- Separation of straight-chain hydrocarbons from the branched-chain hydrocarbon
- Medical monitoring in cancer treatment
- Air separation
- Removal of heavy metals
- Water purification
- Petrochemical cracking
- Chemical sensors in industrial process
- Catalysis: in proton transfer






