Table of Contents
ToggleGas chromatography mass spectrometry (GC-MS) is an analytical technique that combines two powerful techniques; gas chromatography and mass spectrometry and is used to separate, identify, and quantify volatile compounds. It is therefore perfect for analyzing the many relatively low molecular weight compounds. Although it can be used with solid, gaseous, and liquid samples, GC-MS analysis is most often used with volatile and semi-volatile compounds.
In Gas Chromatography (GC), the chemical constituents of a sample mixture are separated, which is then used to identify the components to ascertain whether or not they are present, as well as how much of each component is present. The information provided by GC detectors is constrained; it is typically two-dimensional and includes the retention time on an analytical column and the detector response. Identification is based on comparing the retention times of the peaks in a sample to standards of well-known substances that were also subjected to the same kind of analysis. Although GC alone cannot be utilized to identify unknowns, hyphenation to mass spectrometry can be quite effective in such a scenario.
The molecular weight and elemental constituents of molecules, as well as their chemical structures, can be determined using the analytical method known as MS, which measures the mass-to-charge ratio (m/z) of charged particles. A GC-MS produces three-dimensional data, including chromatograms for qualitative and quantitative examination as well as mass spectra for confirming the identity or identifying chemical compounds.
How does Gas Chromatography-Mass spectrometry work?
The first step in the analysis is the gas chromatograph, where a capillary column coated with a stationary (liquid or solid) phase separates the sample into its constituent components after efficiently vaporizing it into the gas phase. An inert carrier gas, such as helium, hydrogen, or nitrogen, propels the chemicals. Depending on its boiling point and polarity, each compound elutes from the column at a different time as components of the mixture are separated. The retention time of a compound is the period of elution. Complex mixtures or sample extracts containing hundreds of different compounds can be analyzed using GC.
The mass spectrometer uses electron or chemical ionization sources to ionize and fragment the components after they left the GC column. Then, accelerated molecules and fragments are sent via the mass analyzer of the device, which is frequently a quadrupole or ion trap. Here, ions are divided into groups according to their various mass-to-charge (m/z) ratios. Either full scan mode, which covers a broad range of m/z ratios, or selected ion monitoring (SIM) mode, which collects data for certain masses of interest, can be used for GC-MS data collecting.
The procedure ends with ion detection and analysis, where fragmented ions can be identified based on their m/z ratios. The amount of the related compound is proportional to peak areas. The gas chromatogram of a complex sample separated by GC-MS will show a variety of peaks, each of which produces a distinctive mass spectrum useful for compound identification. Unknown compounds and target analytes can be located and measured using broad commercial mass spectral libraries.
GC-MS principle
Gas chromatography can only separate mixtures that are volatile within the instrument’s working temperature range. This enables the separation of several samples, providing they do not break down in the system, and ovens may be temperature-programmed to run from ambient to high temperatures. Stable stationary phases are available for use up to 400°C. An inert gas (such as helium) is used to carry the heated gases through a column. Although the separated components can be categorized based on their retention durations or by chromatographing spiked samples, other methods are needed for distinctive identification.
Mass spectrometry is a crucial tool for the identification of compounds. Therefore, the separated compounds enter the MS as they exit the column aperture of GC. Either electron impact or softer methods like chemical ionization can ionize a substance. This is highly helpful in identifying biological species that are less stable.
Instrumentation of GC-MS
The effluent gases from the gas chromatograph have a pressure that is quite near to that of the atmosphere, and they contain both the carrier gas and the separated components. It is possible to perform detection using a flame ionization detector (FID) or one of the other detectors available on the GC. This can be accomplished by separating the effluent stream at the column exit, which enables the majority of the sample to be introduced into the mass spectrometer. After that, it is required to lower the pressure to the operating pressure of the mass spectrometer, which is somewhere in the neighborhood of 108 Nm2.
With a capillary GC column, the flow of carrier gas is kept to a minimum, and the effluent can be supplied directly into the mass spectrometer via a fine capillary. It is necessary to have an interface between the mass spectrometer (MS) and the gas chromatograph (GC) when using a packed GC column. It’s possible that this is a porous tube separator, but it could also be a jet separator. In both of these processes, the low-mass carrier gas, which is typically helium, diffuses away and is evacuated by pumps, while the bigger sample molecules continue through into the MS. Figure illustrated below displays a whole GC-MS system in its entirety.
The temperature of the interface needs to be maintained at the same level as the temperature of the GC outlet, and this is typically accomplished by enclosing the interface in the GC oven.
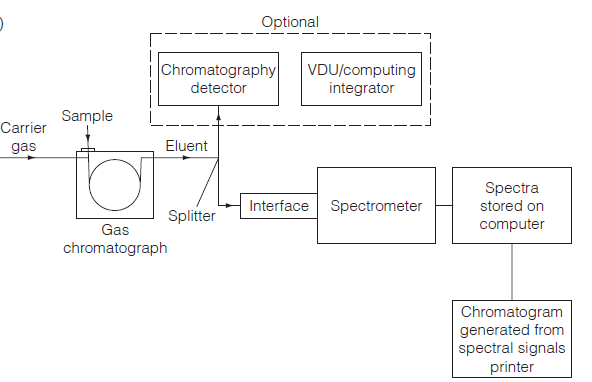
The majority of mass spectrometers can be utilized for GC-MS work; however, those equipped with a quadrupole analyzer are preferred because of their capacity to scan rapidly. The GC detector and data system will record the chromatogram, but it can also be made by constantly measuring the total ion current (TIC) for the ions made as a function of the amount of time that has passed. This total ion current chromatogram is the same as the one from the GC instrument, and it may be able to find other solutes as well. By choosing a certain mass/charge (m/z) ratio, selected ion monitoring (SIM) can be used to find a specific ion. The GCMS instrumentation is illustrated as:
Besides these, the components of GC-MS instrumentations are:
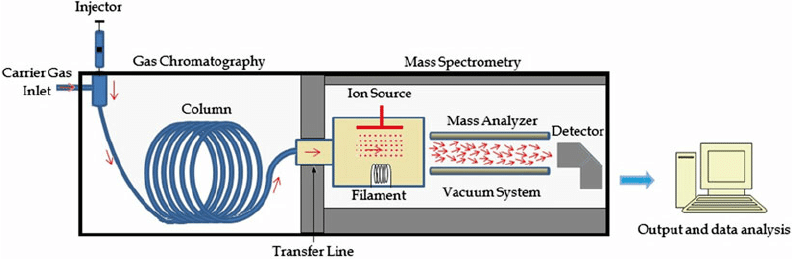
Components of Gas-Chromatography
- Carrier gas system (eg. helium, nitrogen, argon, hydrogen)
- Sample injection system
- Oven
- GC-columns (eg. Capillary and packed)
- Detectors ( Flame ionization detector, thermal conductivity detector, Electron capture detector, Mass detector)
- Read-out device
Interface
- Direct interface
- Jet interface
- Biemann concentrator
Components of mass spectrometer
- Sample input system
- Ion source
- Mass analyzer ((eg. Quadrupole mass analyzer, Time of flight mass analyzer, Magnetic sector analyzer)
- Detectors (eg. Electron multiple detectors, Faraday cup detectors, Array detectors)
- Signal processor
- Readout device
How Does GC-MS instrument work?
- After the GC separates the sample mixture, the analyte molecules are eluted into the mass spectrometer for detection. The carrier gas constantly moves them through the GC and into the MS, where they are removed by the vacuum system.
- The sample is inserted into the GC manually or with an autosampler. It then goes into the carrier gas through the GC opening. If the sample is a liquid, it is turned into a gas at the heated GC inlet and then sent to the analytical column.
- The so-called “analytes” are separated by either being absorbed by a solid stationary phase (in the case of less volatile gases) or being partitioned between the mobile phase (carrier gas) and the liquid stationary phase (which remains in the column). The typical GC-MS analysis employs a short (10-30 m) column with a thin (0.1-0.25 mm internal diameter) liquid stationary phase.
- After separation, which doesn’t need total baseline resolution for GC-MS analyses unless the analytes are isomers, the neutral molecules flow into the mass analyzer through a heated transfer line.
- Mass spectrometer EI ionizes neutral compounds. EI creates radical cation molecular ions by accelerating a filament-produced electron to 70 eV and knocking an electron out of the molecule. High-energy ionization can fragment a molecule ion and waste energy. Bond breakage(s) can produce radical or neutral molecule loss and chemical rearrangements. The molecule ion is the heaviest while fragment ions vary in mass.
- Following this, the mass analyzer is used to separate the ions based on their m/z.
- The mass analyzer separates the ions based on their m/z, and once they arrive at the ion detector, the signal is amplified by an electron multiplier (for the majority of low-resolution MS) or a multi-channel plate (for the majority of HRMS instruments) depending on the instrument. Each data point receives a chromatogram and a mass spectrum through the acquisition program on the computer that records the signal.
Types of GC-MS
Various types of analysis require various detection abilities. Different types of mass spectrometers may be needed for different analyses, even if the gas chromatography system remains the same, depending on the amount of selectivity and sensitivity required. Generally, there are 3 types of GC-MS:
- Single quadrupole GC-MS: When gas chromatography is coupled with a mass spectrometer that consists of only one quadrupole, the resulting technique is frequently referred to as GC-MS. Because these systems may be operated using either targeted selected ion monitoring (SIM) or untargeted full scan acquisition, GC-MS is an excellent choice for the routine analysis of samples in situations in which either targeted or untargeted analysis is required. This makes GC-MS particularly well suited for use in situations where either targeted or untargeted analysis is required. Typical uses include screening for pesticides in food and environmental samples, screening biological samples for illegal drugs, and screening water samples for volatile organic chemicals.
- Triple quadrupole GC-MS/MS: GC-MS/MS is an abbreviation that stands for gas chromatography that has been integrated with a triple quadrupole mass spectrometry system. The triple quadrupole mass spectrometer is most useful for performing analyses requiring the highest possible level of sensitivity since it offers a better level of selectivity. When attempting to quantify the amount of pesticides in food or environmental toxins, this situation frequently arises. The selective reaction monitoring (SRM) mode is the default setting for GC-MS/MS systems when they are being used. The SRM’s strong selectivity helps limit interference from background ions and produces a high signal-to-noise ratio, both of which contribute to the superior detection capacity of the instrument.
- HRAM GC-MS/MS: Combining a GC system with a high-resolution accurate mass (HRAM) mass spectrometer allows for the comprehensive characterization of samples in a single study, including the discovery, identification, and quantification of compounds with high confidence. These GC-MS/MS instruments combine the quantitative ability of a triple quadrupole GC-MS/MS with the full-scan, high-precision HRAM capabilities of the most effective mass spectrometers. Applications that require precise targeted analysis and reliable identification of unknown compounds are well suited to these systems.
Advantages of GC-MS
The analyte needs to have a considerable vapor pressure between 30 and 300 degrees Celsius in order to be analyzed by GC. GC provides insufficient evidence regarding the nature of the compounds that were identified. The matching of the retention times is the basis for the identification, which may contain errors or be misleading. The mass of a particular particle, denoted by GC-MS as Da, is equated to the number of electrostatic charges, denoted by z, that the particle carries, e. The unit of measurement for m/z is DA/e. Electron impact (EI) and chemical ionization (CI) are two techniques that are frequently used in GCMS.
The main features of an enhanced molecular ion, are improved confidence in the sample identification, significantly increased range of thermally labile and low volatility samples suitable for analysis, significantly faster analysis, improved sensitivity particularly for compounds that are difficult to analyze, and the many other features and options provide compelling reasons to use the GC-MS in a broad range of areas. These reasons include enhanced molecular ion, improved confidence in the sample identification, significantly increased range of samples amenable for analysis that are thermally labile.
Limitations of GC-MS
- The fundamental drawback of GC is that it can only be used to distinguish compounds that are either volatile or thermally stable.
- High cost
- Complex sample preparation: In order to properly prepare samples for GC-MS analysis, including extraction and cleanup steps, it can take a while and call for specialized tools and knowledge.
- Limited sensitivity: Although some GC-MS may have excellent sensitivity, the technology might not be enough for the analysis of complicated materials or the detection of trace pollutants.
- Interferences: Some compounds in the sample may affect how accurately the analysis is performed and cause false positives or false negatives.
- Limited structural information: While GC-MS can offer comprehensive details on a compound’s molecular weight and elemental composition, it might not be able to offer details about the molecular structure or particular functional groups that are present.
- Matrix effects: The precision and dependability of the GC-MS analysis might be impacted by the presence of additional substances in the sample, collectively referred to as the matrix. Careful sample preparation and the use of internal standards can help reduce these matrix effects, although they still may introduce some errors.
- Limited selectivity: Although GC-MS has a high degree of sensitivity and specificity, it may not be able to differentiate between isomers or structurally similar molecules. Issues with GC-MS can make it difficult to identify and quantify certain compounds.
- Limited linear range: GC-MS can only reliably detect concentrations of a drug within a particular range due to its limited linear range. If the compound concentration is outside of this range, the results may not be correct.
- Recovery of the sample’s individual components is not possible.
- It is important to use caution when employing the hydrogen gas that causes the flame.
- Except for MS, all of the detectors utilized in the GC are destructive.
Application of GC-MS
- Medicine: Several congenital metabolic illnesses can be detected with the help of GC-MS screening techniques. Patients with hereditary metabolic abnormalities can be diagnosed by testing their urine for the presence of trace chemicals. Oils in ointments, creams, and lotions are also detectable by this instrument.
- Biological and pesticide detection: Blood and urine can be tested for the presence of drugs using GC-MS. This includes anesthetics, anticonvulsants, antihistamines, sedative-hypnotics, opioids, and anti-epileptic medications.
- Pharmaceutical industries: The pharmaceutical industry makes extensive use of GC-MS in the areas of analytical development, quality control, quality assurance, production, and pilot plants for API, bulk medicines, and formulations.
- Clinical toxicology: GC-MS is extensively employed in clinical toxicology to identify toxins and venoms.
- Environmental monitoring: The gas chromatograph-mass spectrometer (GC-MS) is now widely used as a method for detecting organic contaminants in the environment.
- Industries: Common applications of GC-MS include regular analysis in the food, environmental, forensics, anti-doping, and consumer products industries to detect volatile compounds with molecular weights typically below 700 amu.
- Forensic Applications: The American Society for Testing Materials (ASTM) specifies that GC-MS be used for fire debris analysis. GC-MS is commonly employed in anti-doping laboratories to detect performance-enhancing drugs like anabolic steroids, and in forensic toxicology to detect poison and steroids in biological materials.
- Food and Fragnance applications: The fatty acids, aldehydes, esters, alcohols, and terpenes present in food and drink can be easily analyzed using gas chromatography-mass spectrometry. It also has the ability to detect spoilage and contamination in food.
- Chemical Welfare: The GC-MS method is utilized by explosive detection systems installed in public locations for the purpose of conducting analyses and locating chemical warfare chemicals.
- Academic research: The GC-MS is an advanced technique that allows for the characterization and identification of newly synthesized or derivatized compounds.






