Table of Contents
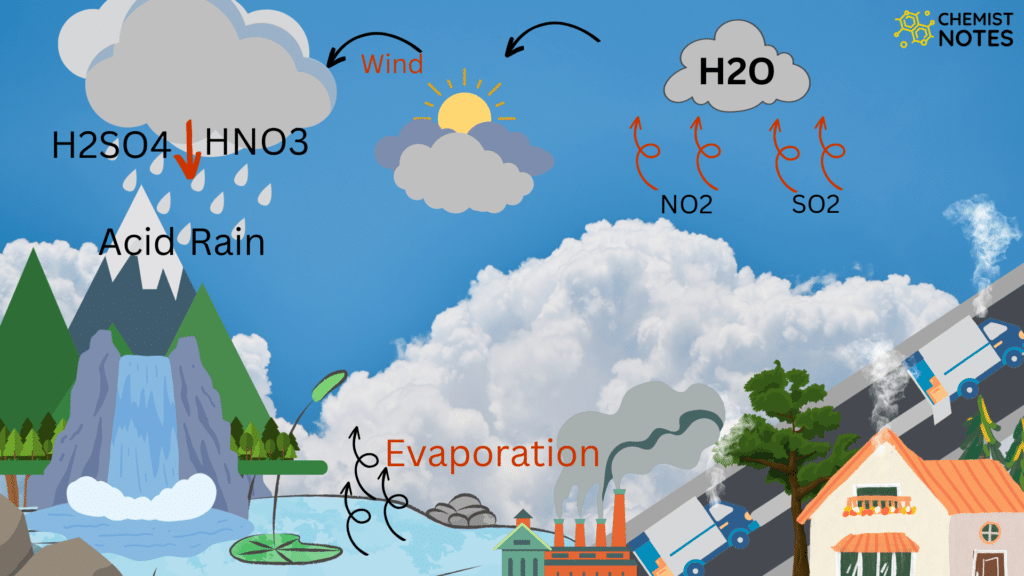
ToggleAcid rain, also known as acid deposition, refers to any type of precipitation with acidic components, such as sulfuric or nitric acid, that falls to the earth from the atmosphere in wet or dry forms. Wet deposition refers to any type of precipitation that takes acids from the atmosphere and deposits them on the earth’s surface. In the lack of precipitation, dry deposition of polluting particles and gases adhere to the earth’s surface through dust and smoke.
Causes of Acid rain
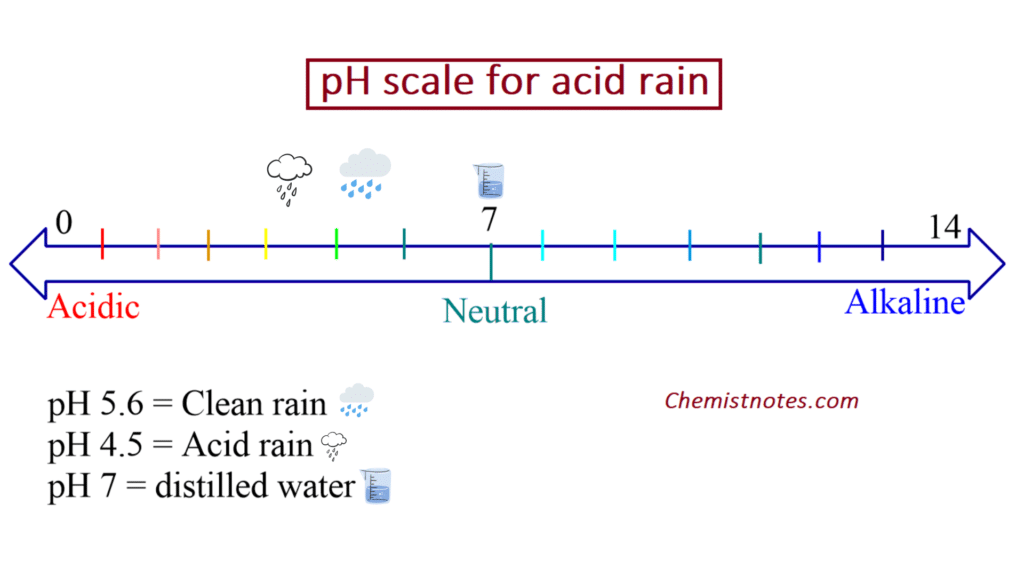
Generally, rain water has a pH of approx. 5.6 due to dissociation of CO2.
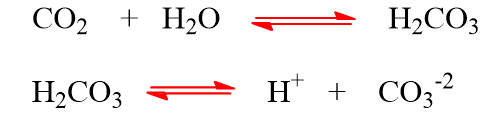
What causes Acid rain?
The pH of the rain water may be decreased below pH 5.6 if the acidic oxides of sulfur and nitrogen get dissolved in it which is called “acid rain“. In fact, acid rain is caused due to oxides of nitrogen (NO2) and oxide of sulphur (SO2, SO3).
Sources of oxides of sulphur (SO3) are:
- Combustion of fossil fuels to generate electricity.
- Petroleum refining
- Roasting of sulphur-containing ores in the furnace.
- sulphur-based industry like manufacturing of H2SO4.
Sources of oxides of nitrogen are:
- Automobile exhaust
- electric discharge during thunder or storms.
These oxides of S and N in presence of air and rain water form acids.
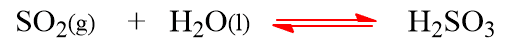
Harmful effects of the acid rain
- Acid rain is harmful to marine life.
- It damages the building structure made of marble, cement, and or of lime, mortar, iron bridges, and statue. This corrosion of stone containing CaCO3 by acid rain is called ‘stone leprosy’.
- Building and sculptural material of marble, limestone, slate, mortar, etc becomes pitted and weakened mechanically by acid rain as the partially soluble suphate and highly soluble nitrates are leached out by rain water.
- It fades the color of the fabrics
- Acid rain reduces soil fertility.
- Acid rain dissolves heavy metals like Cu, Pb, Hg, Al, etc. from the soil and rocks. The ions of these metals leached from the soil and rocks then percolated down into the ground water and may cause serious pollution in ground water.
Control Measures of acid rain
- The scrubber should be used to reduce the emission of SO2 during coal burning.
- Powered limestone (CaCO3) should be injected into the power plant boiler of the furnace along with coal. The quicklime obtained from CaCO3 reacts with SO2 produced by combustion.
- It is better to install a sulphuric acid plant near a metal ore refining site in order to cut SO2 emissions.
- Public awareness should be launched regarding the pollution effect of acid rain.
Related video
What is acid rain?
Acid rain, refers to any type of precipitation with acidic components, such as sulfuric or nitric acid, that falls to the earth from the atmosphere.
Is acid rain real?
Acid rain is real.
what is the ph of acid rain?
pH of acid rain is around 5.5.