Table of Contents
ToggleAldehydes and ketones are compounds that contain a carbonyl group (C=O), and therefore, these compounds are collectively referred to as carbonyl compounds. Aldehydes are the compounds that have the general formula RCHO, and ketones are the compounds having the general formula RR’CO. The groups R and R’ may be alkyl or aryl groups.
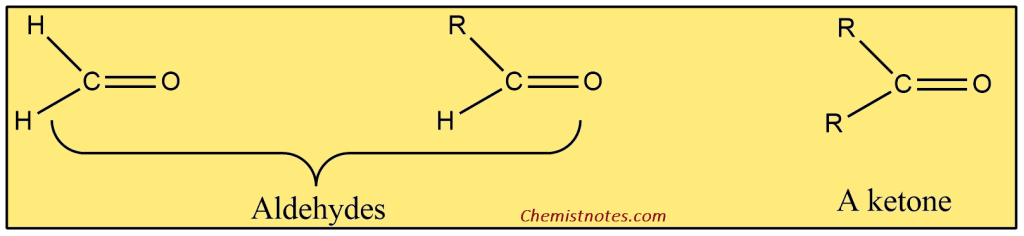
In fact, the carbonyl group determines the chemistry of aldehydes and ketones. It is not surprising to find that aldehydes and ketones resemble each other closely in most of their properties. However, there is a hydrogen atom attached to the carbonyl group of aldehydes, and there are two organic groups attached to the carbonyl group of ketones. This difference in structure affects their properties, mainly the characteristics reactions of carbonyl compounds.
Structure of Carbonyl Group
Let us examine the structure of the carbonyl group. The carbonyl carbon atom is in sp2 -hybridized state, and it is joined to three other atoms by σ-bonds. These bonds lie in a plane and have bond angles of 120o. The remaining p-orbitals of carbon and oxygen atoms overlap sidewise to form a π-bond. Thus the double bonds of the carbonyl group are made σ-bond and one π-bond.
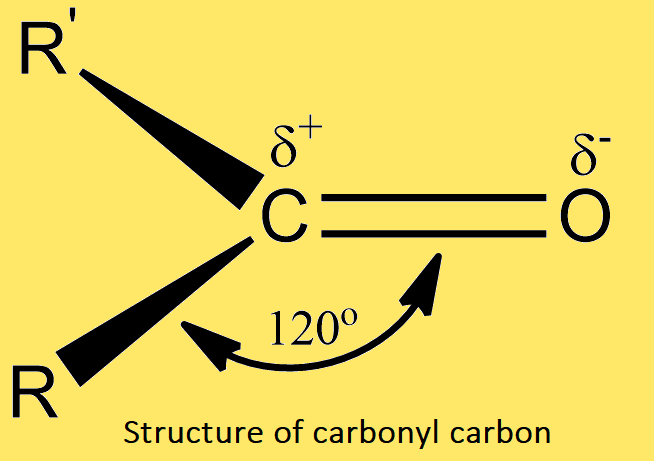
The part of the molecule immediately surrounding the carbonyl carbon is flat, and thus the carbon, oxygen, and the other two atoms or groups attached to carbonyl carbon lie in the same plane. The oxygen atom is more electronegative than carbon, and hence the p electron cloud is displaced towards oxygen. Consequently, the carbon atom of the carbonyl group becomes slightly positive and oxygen slightly negative.
Many experimental observations also support the above structure of the carbonyl group. The electron diffraction and spectroscopic studies show that carbon, oxygen, and the other two atoms attached to carbonyl carbon lie in the same plane, and the bond angles are approximately 120o. The high dipole moments (2.3 to 2.8D) also indicate the ionic character of the carbon-oxygen bond.
Nomenclature of Aldehyde and Ketone
Common system
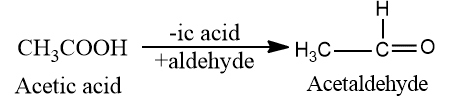
Aldehydes: In the common system, the names of aldehydes are given according to the name of the corresponding carboxylic acid, which they form on oxidation. The suffix “-ic” acid of the name of the corresponding acid is replaced by the suffix “-aldehyde.”
Branched-chain aldehydes are named as the derivatives of straight-chain aldehydes. The position of substituents is indicated by Greek letters, -α, -β, and -γ, etc. The letter -α is used for the carbon attached to the -CHO group.
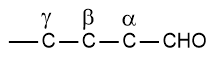
Ketones: In the common system, the names for ketones are obtained by naming the two alkyl groups attached to the carbonyl group in alphabetic order and adding the word ketone. The first member of the series is commonly called acetone.
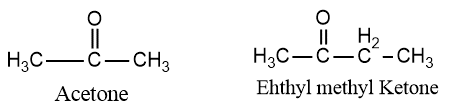
IUPAC system
Aldehydes:
- In the IUPAC system aldehydes are named alkanals.
- The name is derived by replacing the -e of the corresponding alkane with -al.
- In order to write the name of the branched chain aldehydes, the longest chain containing the -CHO group is selected as the parent chain.
- The parent chain is then numbered in such a way that the -CHO group gets the lowest number (1).
- Since the position of the aldehydic group is always 1, It is normally not indicated in the final name of the compound.
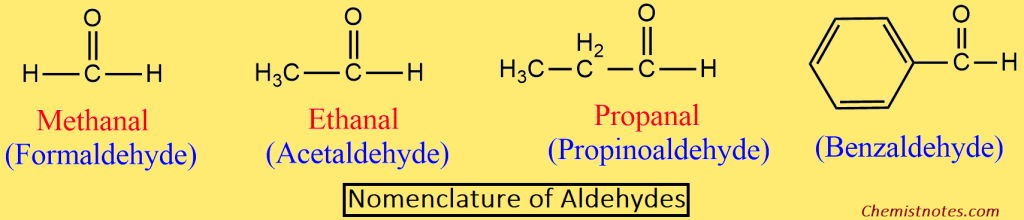
Ketones:
- In the IUPAC system, ketones are named alkanones.
- At first, the longest chain containing the carbonyl group is selected as the parent chain, and the name is derived by replacing the -e of the corresponding alkane with -one.
- In order to write the name of higher ketones, the parent chain is numbered in such a way that the carbonyl carbon gets the lowest number.
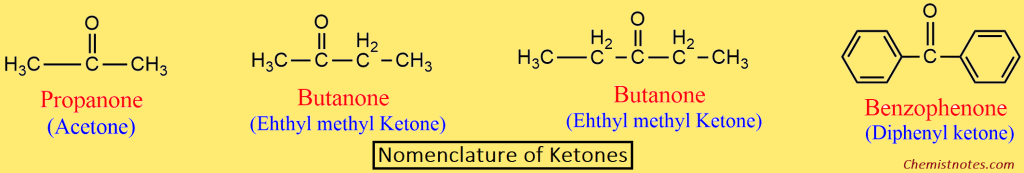
Physical Properties of Aldehyde and Ketone
Aldehydes and ketones are polar compounds having sufficient intermolecular dipole-dipole interactions between the opposite ends of C=O dipoles. Therefore, aldehydes and ketones have higher boiling points that hydrocarbons of comparable molecular masses. But they are not able to form intermolecular hydrogen bonding, and hence boiling points of aldehydes and ketones are relatively lower than alcohols and carboxylic acids of comparable molecular weight.
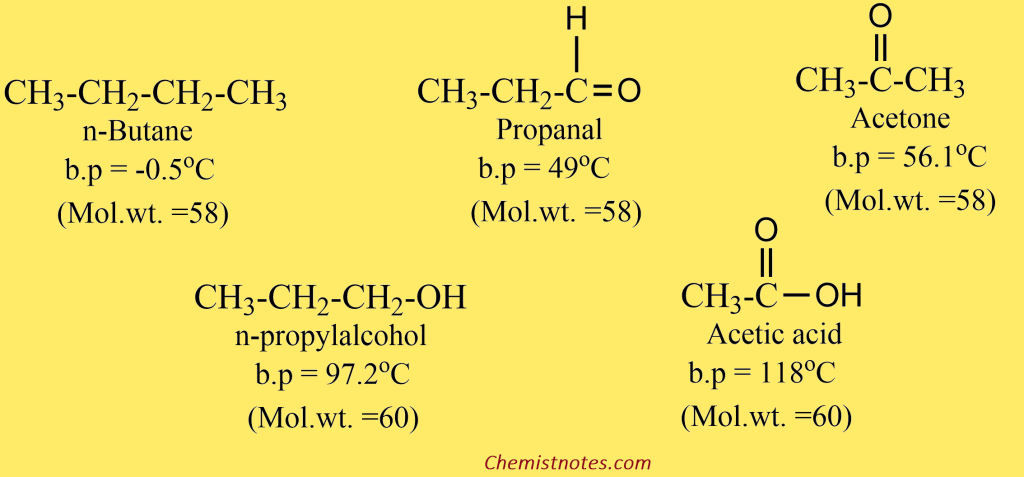
The polar carbonyl group of aldehydes and ketones is capable to form strong hydrogen bonds with water molecules. As a result, lower aldehydes and ketones are miscible with water. Acetone and acetaldehyde are soluble in water in all proportions. The first member of aliphatic aldehyde, formaldehyde, is a gas. Its aqueous solution, known as formalin, or its solid polymers i.e. paraformaldehyde (CH2O) or trioxane (CH2O)3 are used for practical purposes.
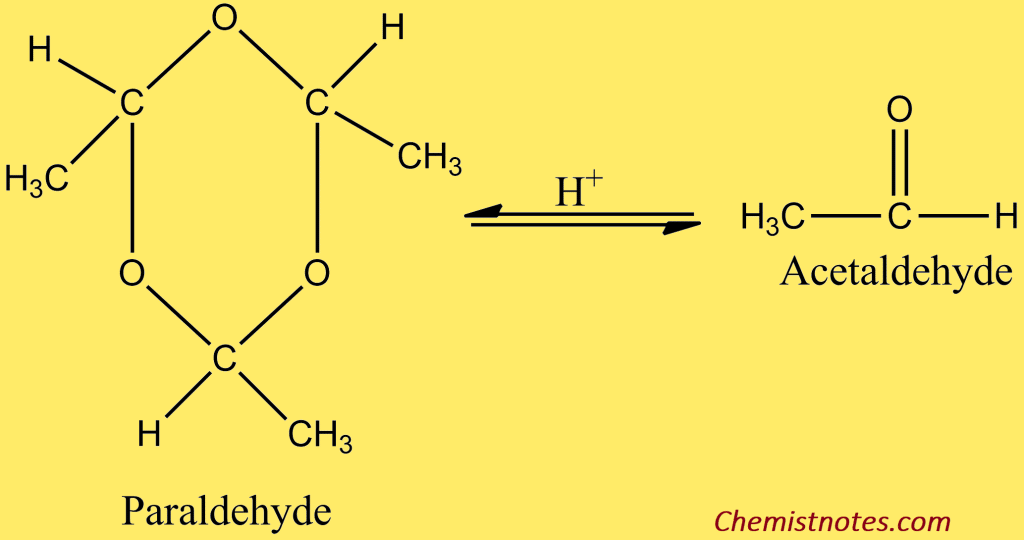
Acetaldehyde is obtained from its higher boiling trimer, i.e. paraldehyde ( boiling point 125oC) by heating with acid.
| Name | Melting point (oC) | Boiling point (oC) | Solubility gm/100 gm water |
| Aldehydes: | |||
| Formaldehyde | -92 | -21 | Very soluble |
| Acetaldehyde | -121 | 20 | ∞ |
| Propanal | -81 | 49 | 16 |
| Butanal | -99 | 76 | 7 |
| Pentanal | -91 | 103 | Slightly soluble |
| Benzaldehyde | -26 | 178 | 0.3 |
| Ketones: | |||
| Acetone | -94 | 56 | ∞ |
| Butanone | -86 | 80 | 26 |
| 2-Pentanone | -78 | 102 | 6.3 |
| Acetophenone | 21 | 202 | Insoluble |
| Benzophenone | 48 | 306 | Insoluble |
FAQs
How are ketones and aldehydes related?
Aldehydes and ketones are compounds that contain a carbonyl group (C=O), and therefore, these compounds are related compounds.
What is an aldehyde functional group?
Functional group contain in aldehyde is carbonyl group C=O.
What is the difference between aldehyde and ketone?
Aldehyde and ketones are different in structure which affects therie properties like aldehyde are quite easily oxidized, and aldehydes are usually more ractive than ketones.






