Table of Contents
ToggleArrhenius theory of ionization or electrolytic dissociation was given by Arrhenius in 1884. He proposed this theory when he came to conclude that the conductivity of the solution is due to the presence of ions in the solution.
Main postulates of Arrhenius theory of ionization
The main postulates of Arrhenius theory of ionization have been described below:
- The molecules of an electrolyte in solution breaks up into two types of charged particles called ions, this process is known as ionization. The positively charged ion is called cation while negatively charged ion is called anion. For example: When NaCl is dissolved in water, it breaks up into Na+ and Cl– ion. The Na+ ion is called cation and Cl– ion is called anion.
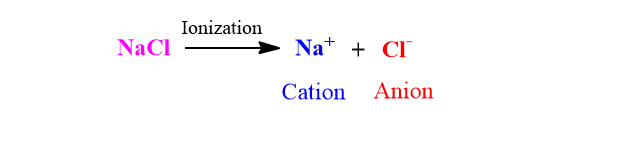
2. The total charge on the cation is equal but opposite to that on the anion so that the solution as a whole is electrically neutral. For example, KNO3 on dissolving in water dissociates as K+ and NO3– . Here, the total charge on the cation and anion is one. Therefore when we consider the solution as a whole, the solution is neutral.
3. The process of ionization is reversible. The ions present in a solution are constantly reuniting and the molecules are dissociating. Thus, there is a state of equilibrium between the dissociated ions and unionized molecules. Such equilibrium is called ionic equilibrium. For example:
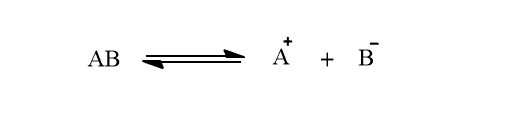
4. Electrical conductivity of electrolytes is due to the migration of ions towards oppositely charged electrodes. i.e cations move towards the cathode and anions move towards the anode.
5. The properties of an electrolyte in solution are the properties of ions produced.
6. The extent of ionization is expressed in terms of the degree of ionization. The degree of ionization is defined as the ratio of no. of moles of ions to no. of moles of undissociated molecules. Mathematically, the degree of ionization can be expressed as:
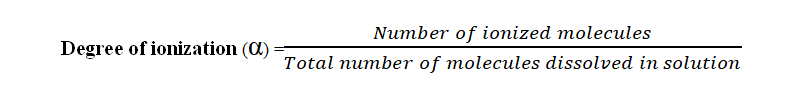
- The degree of ionization is denoted by α.
- The value of degree of ionization for strong electrolyte is almost one and that for weak electrolyte is always less than one.
Limitations of arrhenius theory of ionization
Although Arrhenius theory of ionization has explained the important concept of ionization for strong and weak electrolytes, it has some limitations which are described below.
- Strong electrolytes are almost dissociated in to its ions even at moderate concentration.Therefore, there is no chance of extistence of ionic equilibrium.
- The value of degree of ionization obtained from conductance measurement and colligative properties, agree well for the weak electrolytes but not for the strong electrolytes.
- The Arrehenius theory does not consider the interionic attraction between the ions. This is the main reason of failure of it in the case of strong electrolytes. In real, there exists certain forces of interaction between the ions.
Later, Debye and Huckel proposed a theory that has tried to overcome the limitations of Arrhenius theory of ionization by considering the effect of force between the ions i.e interionic forces.
Arrhenius theory of ionization in hindi
References
- P.W. Atkins & J.D. Paula, Elements of Physical Chemistry, 4th Edition, Oxford University Press, 2010
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and CompanyLtd., New Delhi, 2012
- M. L. Sharma & P. N. Chaudhary, A Textbook of B. Sc. Chemistry (Volume II), 2nd Edition, Ekta Books Nepal, 2007