Table of Contents
ToggleThe preparation of colloids that can be carried out using several techniques is well-discussed below:
Colloids are those particles that are intermediate between true solution and suspension and have a diameter of 1 to 100nm. Considering that the sizes of colloidal particles are intermediate between those of molecules and those of macroscopic bulk phases, it seems reasonable to assume that the mechanism of colloidal formation could take one of two paths: either breaking large pieces down to the required size (a process known as comminution or dispersion) or starting with a molecular dispersion and building up the size by aggregation (i.e. condensation). There are so two approaches to the preparation of colloids, namely:
- Dispersion Methods
- Condensation method
Dispersion Method
As the name implies, colloidal-sized particles are formed by disintegrating a large amount of hydrophobic material in the dispersion or disintegration methods.
Formation of colloids from Dispersion methods can be obtained by:
- Mechanical dispersion
- Electro-dispersion
- Ultrasonic dispersion
- Peptization
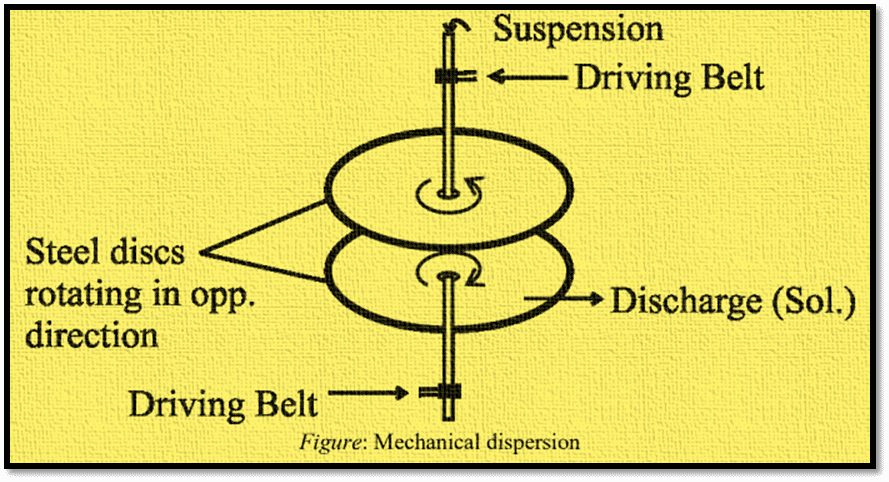
i) Mechanical dispersion
This technique involves feeding a colloidal mill with both the solid and the liquid. Two steel plates that are almost touching and revolving quickly in opposite directions make up the colloidal mill. The liquid is then used to disperse the colloidally-sized solid particles. This process is used to create printing inks and colloidal graphite, food products, cement, pharmaceutical products, and so on.
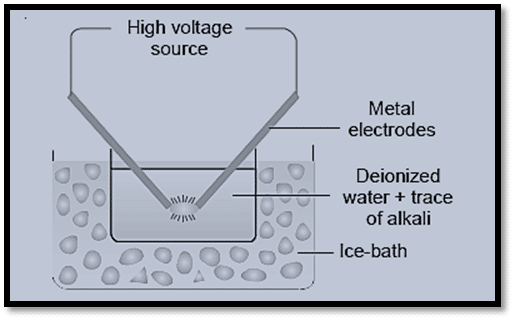
ii) Electro-dispersion
Electro-dispersion techniques are used to create colloidal solutions of various metals, including gold, silver, platinum, etc. Under the surface of water containing some stabilizing agent, such as a trace of alkali, an arc is created between the metal electrodes. Placing the container in a cold bath allows the water to cool. Some of the metal is vaporized by the arc’s high heat, and some of it then condenses in the cold water.
iii) Ultrasonic dispersion
Ordinarily, ultrasonic waves are used to describe high-frequency sound waves. The solution containing the bigger particles is subjected to such waves. They break down to form a colloidal solution.
iv) Peptization
Peptization is the process by which a precipitated substance is dispersed into a colloidal solution by an electrolyte in the solution. The electrolyte used is called a peptizing agent.
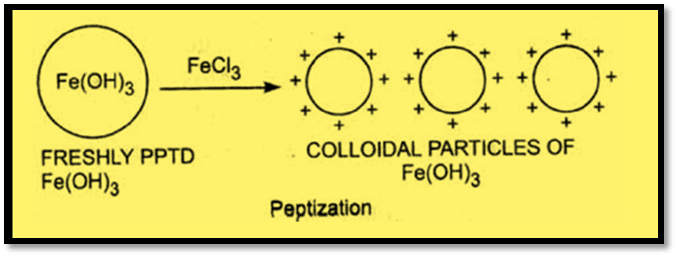
For example, Ferric hydroxide is converted into a reddish-brown colloidal solution when ferric chloride is supplied to a precipitate of ferric hydroxide. This results from the precipitate preferentially adsorbing electrolyte cations. Fe3+ ions from FeCl3 are adsorbed by Fe(OH)3 particles when FeCl3 is introduced to Fe(OH)3. As a result, the Fe(OH)3 particles acquire a positive charge and begin to repel one another, producing a colloidal solution.
Condensation Method
These techniques condense smaller dispersed phase particles appropriately to produce colloidal particles of the desired size. Some of the condensation techniques are:
i) Oxidation
By bubbling oxygen (or any other oxidizing agent, such as HNO3, Br2, etc.) through a solution of hydrogen sulfide in water, one can produce a colloidal solution of sulphur.

ii) Reduction
By adding a suitable reducing agent, such as formaldehyde, hydrogen peroxide, phenyl hydrazine stannous chloride, etc. to the aqueous solution of metal salts, some metals, such as silver, gold, and platinum, can be produced in colloidal form.
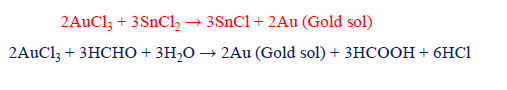
iii) Hydrolysis
Numerous salt solutions can be quickly hydrolyzed by heating diluted solutions of the salts that make up those solutions. For example, ferric hydroxide and aluminum hydroxide sols can be made by boiling diluted solutions of the respective chlorides.
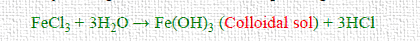
iv) Double decomposition
By passing hydrogen sulfide through a cold solution of arsenious oxide in water, one can form an arsenic sulphide sol.
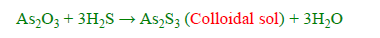
v) Excessive cooling
By freezing a water solution in the solvent, one can create a colloidal solution of ice in an organic solvent like ether or chloroform. Water molecules that can no longer be retained in solution combine on their own to create colloidal-sized particles.
vi) Exchange of solvent
It is possible to generate a colloidal solution of some elements, such as sulfur and phosphorus, by adding more water than their alcoholic solution is soluble in. For instance, adding a sulfur solution in an alcoholic form to water produces a milky colloidal sulfur solution.
vii) Change of physical state
Sols of elements like mercury and sulfur are made by passing their vapors through cold water that has been stabilized with an appropriate agent, like ammonium salt or citrate.