Can we measure the potential difference across electrified interfaces i.e a single electrode/electrolyte interface? Actually, the answer is “NO” and we will discuss why this is impossible. Let’s explain the process of measurement.
Measurement of potential difference across electrified interface
In order to measure potential differences, various potential measuring instruments are available such as Potentiometer, Electrometer, etc. We know all these devices have two metallic terminals which must be connected to the two points between which the potential difference is to be measured.
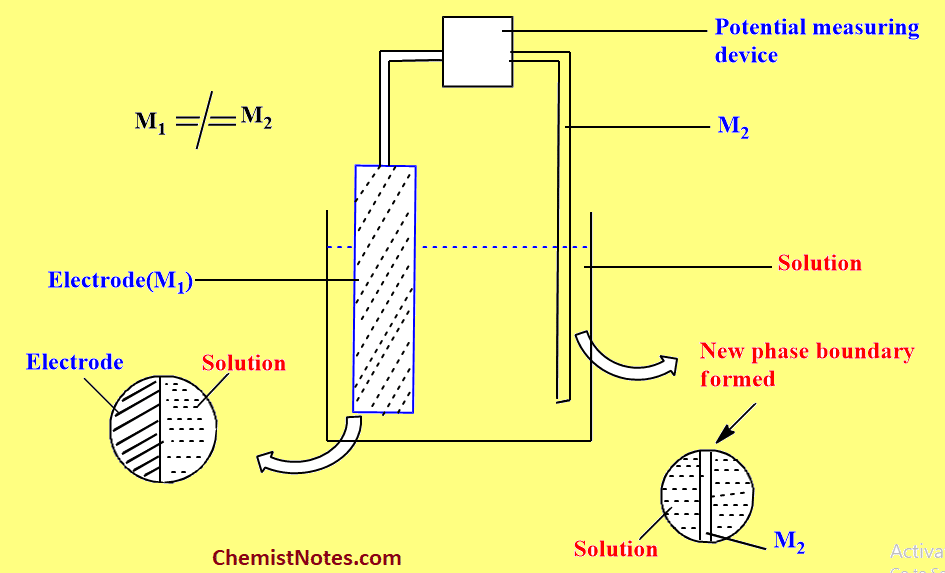
One terminal is connected directly to the electrode and another terminal must be connected to the other phase i.e electrolyte, the potential of which is to be measured, as shown in the figure.
The immersion of the second connecting wire(M2) in the electrolyte inevitably produces a new phase boundary i.e the M2/Solution interface. At this interface, there must be a second double layer and a second metal/solution potential difference i.e PDM2/S which can not be avoided. Therefore, the formation of a second double layer and a second potential difference is the major problem.
Actually, we plan to measure one potential difference PDM1/S but during measurement, one additional potential difference PDM2/S is also measured. Therefore, the final measurement is the sum of at least two potential differences. Hence it is impossible to measure the value of a single metal/solution potential difference.
In summary, the potential difference across one electrode/electrolyte interface can not be measured but the potential difference across a system of interface or a cell can be measured instead.






