Table of Contents
ToggleThe electrified interface, popularly known as the Electrical double layer, consists of a metal and suitable electrolytes, the physical contact between them leads to the development of charges on the interfacial region. The brief introduction and its development have been discussed in this blog.
Electrified interface or Electrical double layer
When a suitable metal is dipped into a liquid electrolyte or solid electrolyte, there is the development of an electrical charge in the region of the phase boundary. This boundary or phase interface with net charge is known as an electrified interface. Due to the development of the interface, an electric potential is developed across the interface.
In other words, it can be stated as ” When two dissimilar phases come into contact, charge separation occurs at the interfacial region or phase boundary. Such charge bearing interface is known as an Electrical double layer.
There are several models to explain the electrified interface but the most popular models are the Helmholtz-Perrin model, Gouy-Chapman model, and Stern model.
Origin of electrified interface
First of all, consider there is no electrode dipped in an electrolyte. In such cases, every particle(ions or solvent) experiences forces that are independent of position and direction in the electrolyte. It means the forces acting on ions are the same in all directions and at every point in the bulk of the electrolyte. Hence, it can be said that there is perfect isotropy and homogeneity.
If a metal electrode is dipped into the electrolyte, the properties of the electrolyte are physically disturbed at the phase boundary of them. It means the force acting on the particles near the boundary is not the same.
Since the forces are different at the phase boundary( contact between metal and electrode) than at the force at the bulk of the electrode, the properties of the phase boundary will be different from the bulk properties. As a result, the presence of an electrode disrupts the uniform properties of the electrolytes in the phase boundary.
We know, the arrangement of particles is affected by the force acting on them. Since new forces exist near the phase boundary or electrode/electrolyte interface, a new arrangement of solvent dipoles and charged particles takes place. As a result, there is the net orientation of solvent dipoles and net excess charge on the electrolyte surface. Hence, the electrolyte side of the boundary is now charged or electrified.
Once the electrolyte side of the phase boundary acquires a net or extra charge, an electric field operates across the boundary, which is experienced by the electrode, and electrons of the electrode migrate towards or away from the boundary depending on the direction of the field. As a result, a charge is induced on a metal that is equal to and opposite to the charge generated on the electrolyte side of the phase boundary.
When charges are separated, a potential difference develops across the interface. The electric forces that operate between the metals and solution constitute the electrical field across the electrode/electrolyte phase boundary. The potential difference across the interface is not large ( about 0.1 v) and thus the field strength is enormous.
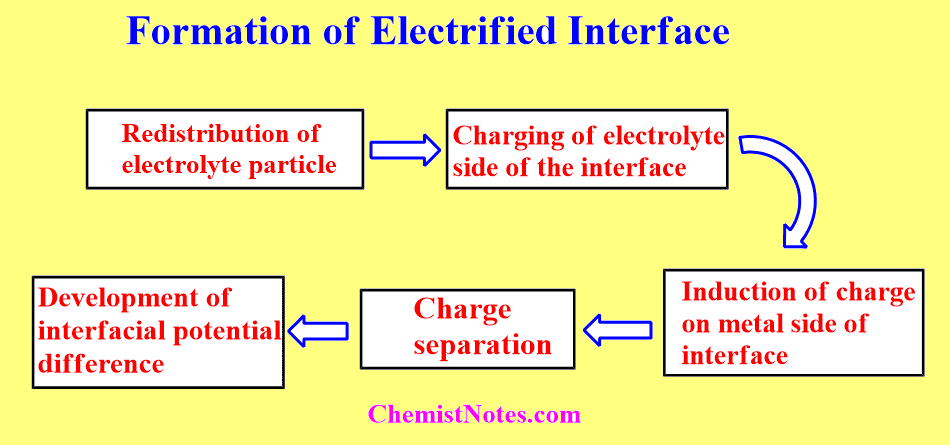
In summary, the formation of an electrified interface can be summarized as:
- Redistribution of electrolyte particles
- Charging of the electrolyte side of the interface
- Induction of charge on the metal side of the interface
- Charge separation
- Development of interfacial potential difference
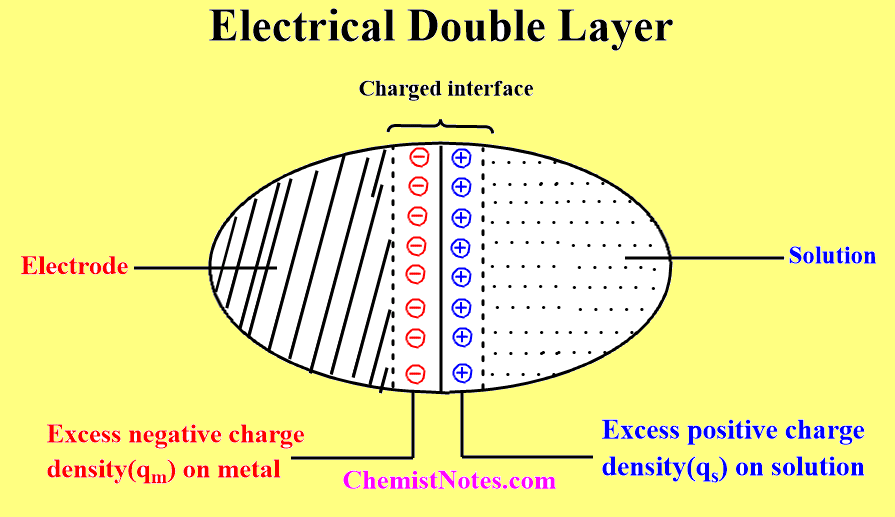
Aspects of electrical double layer
There are two important aspects of the double layer that forms at junction two phases having charged particles.
- Electrical aspect
- Structural aspect
Electrical aspect
The electrical aspect of the double layer deals with the magnitude of the excess charged densities in each phase. It also covers the fluctuation of potential with the distance from the interface.
Structural aspect
The structural aspect is concerned with determining how the particles of the two phases are organized in the interphase area so that the interphase is electrified. Both the electrical aspect and structural aspects are intimately related to each other.
The charge or potential difference is characteristic of the particular structure and vice-versa.






