Table of Contents
ToggleSorensen in 1909, introduced the term pH – value to express H+ ion concentration. The scale on which pH values are computed is called the pH scale. As hydrogen ion concentration can vary within wide limits usually from 1 mole/liter (as in 1 M-HCl) to 10-14 mole/liter (as in 1M-NaOH) in aqueous solutions, therefore, the pH values vary from 0-14. The complete pH scale is:
mathematically,
pH = -log10[H+]
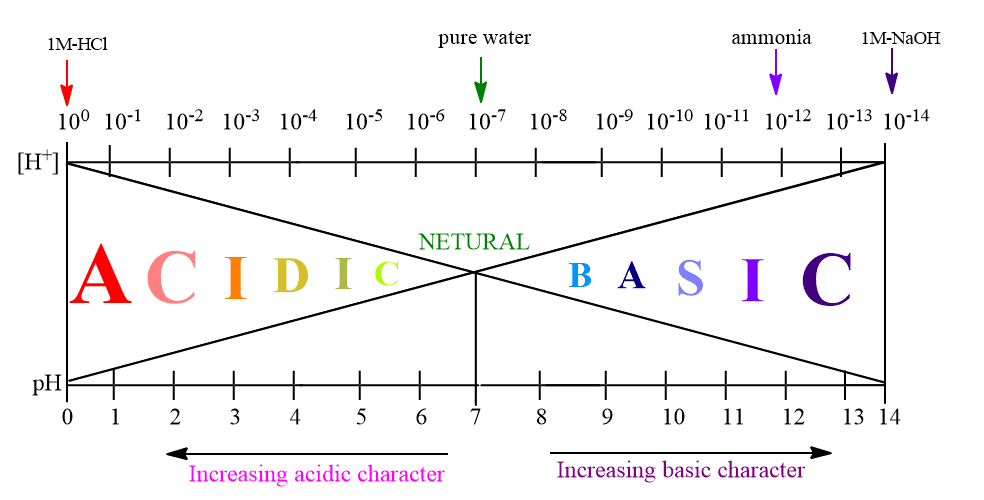
It may be noted that solutions having a pH between 0 to 2 are strongly acidic, solutions having pH of 4 are moderately acidic; and solutions having pH between 4 to 7 are weakly acidic. Similarly, solutions having pH values between 7 to 10 are weakly basic.
Solutions having pH between 10 to 12 are moderately basic, and between 12 to 14 are strongly basic, solution having pH value of 7, are neutral in nature.
pH value of the solution
The pure water dissociates to a very small extent so that we have the equilibrium,

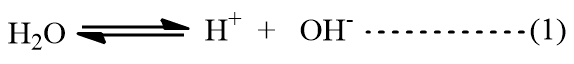
For this equilibrium, the equilibrium constant is given by the expression
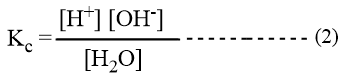
where kc is the ionization constant.
Since water is found to be poorly ionized, the concentration of undissociated molecule H2O can be regarded as constant. Therefore, from the above expression (2) we have;
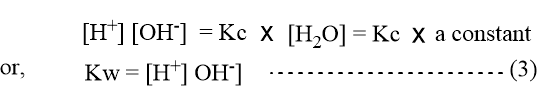
Where Kw is called the ionic product of water. When the concentration of [H+] and [OH–] ions in water are expressed in mole per liter the value of Kw found experimentally is 1.0 × 10-14 at 25oC. Therefore,
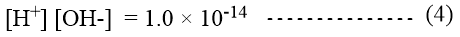
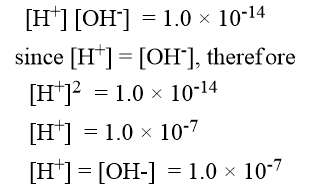
In an acidic solution, however, the concentration of H+ ions must be greater than 1.0 × 10-7 mole per liter. Similarly, in a basic solution, the concentration of OH– ions must be greater than 1.0 × 10-7 mole per liter.
Hence;
For neutral solution = [H+] = [OH–] = 10-7
For acidic solution = [H+] > [OH–], i.e, [H+] > 10-7
For basic solution = [H+] < [OH–], i.e, [H+] < 10-7
Thus, acidity or basicity of a solution can be expressed in terms of its hydrogen ion concentration, [H+].
[H+] = 10-pH
where [H+] is the concentration of hydrogen ion in mole per liter. The pH of a solution may be defined as the negative power to which 10 must be raised in order to express the hydrogen ion concentration of the solution.
Just as pH is used to express hydrogen ion concentration, in the same manner, pOH is used to indicate hydroxyl ion concentration.Thus,
pOH = -log10[OH–]
Relation Between pH and pOH
we know that,
Kw = [H+] [OH–]
Taking the logarithm of both sides, we get
log10 Kw = log10[H+] + log10[OH–]
or, -log10Kw = -log10[H+] – log10[OH–]
or, PKw = pH + pOH
or, 14 = pH + pOH
FAQs
what is the pH value of water?
pH value of water is 7.
What is the pH value of vinegar?
The pH value of vinegar is 2-3.
What is the pH value of blood?
The pH value of blood is 7.35-7.45.
What is the pH value of baking soda?
The pH value of baking soda is 8.3.
what is the pH value of HCl?
The pH value of HCl is 1.
What is the pH value of bleach?
The pH value of bleach is 11-13.
Which pH value indicates the most basic solution?
pH value of 12-14 indicates the most basic solution.
What is the pH value of urine?
pH value of urine is 4.6- 8.0.
What is the pH value of ethylene glycol?
The pH value of ethylene glycol is 5.5-8.0.
What is the pH value of milk?
The pH value of milk is 6.
What is the pH value of lemon?
The pH value of lemon has around 3.
What is the pH value of tap water?
The pH value of tap water is 7.
What is the pH value of tomato juice?
The pH value of tomato juice is 4.05- 4.65.
At which pH values is lipase likely to be denaturated?
lipase likey be denaturated by the time it reaches a pH of 12.
At which pH value do both pepsin and trypsin function.
pepsin function at pH of about 1.5 and trypsin function at pH of about 8,