Table of Contents
TogglePotentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. The definition, the principle involved, curves, examples, and advantages of this titration have been discussed in this post.
Define potentiometric titration
The titration in which the equivalence point is detected by measuring the change in the potential of a suitable electrode after coupling it with a standard reference electrode during the course of the reaction is called potentiometric titration.
For potentiometric titration, an electrochemical cell consisting of two halves cell is required.
- An indicator half cell that potential of which depends on the concentration of an active ion in the cell. The indicator electrode is prepared from the electrolytic solution undergoing titration by inserting a suitable metallic rod. But, the electrode should be an ion-selective electrode.
- A reference half cell the potential of which remains constant. Generally, a saturated calomel electrode is commonly used as the reference electrode.
Principle of potentiometric titration
In all types of titration like acid-base titration, redox titration, precipitation titration, etc. one of the solutions is taken in the titration flask and the other solution is added to it from the burette. As a result, the concentration of some ions in the titration flask keeps on decreasing.
If an electrode reversible with respect to this ion, also known as indicator electrode, is set up in the titration flask or beaker is combined with a calomel electrode as reference electrode, the emf of the cell will keep on changing on adding solution from the burette and show a sudden change in the value when the reaction is complete i.e endpoint reaches. The equivalence point is indicated by the sharp change in the electrode potential during the titration.
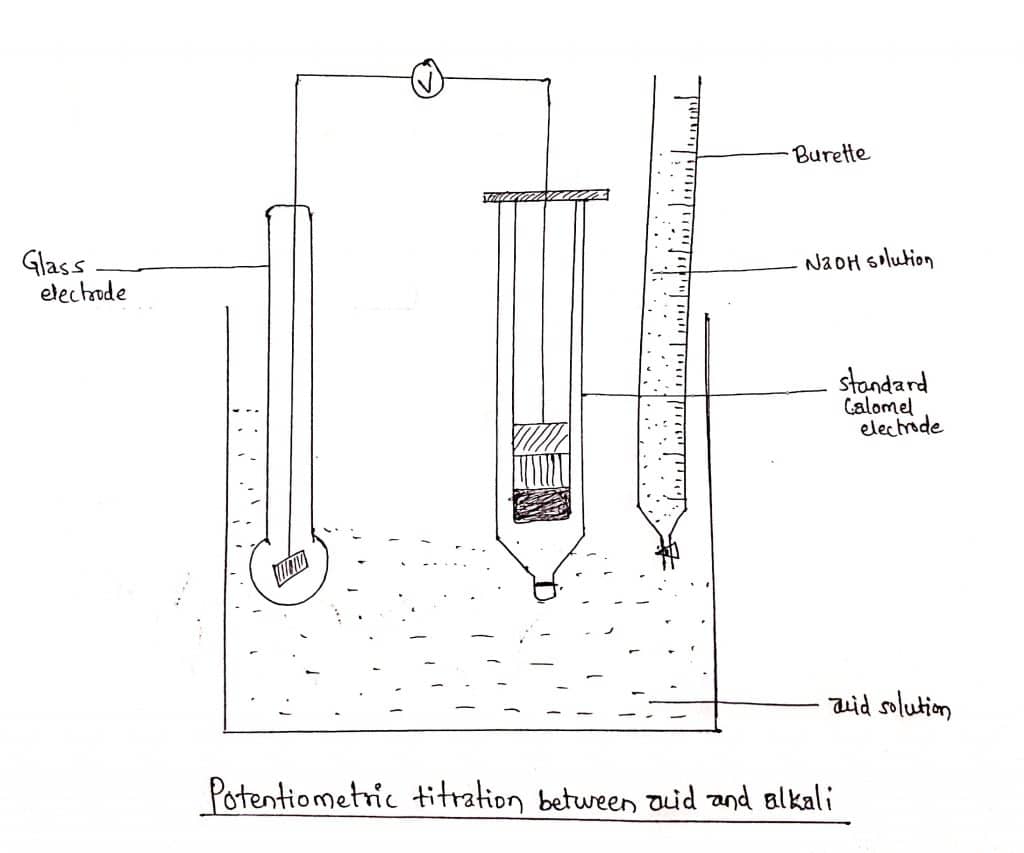
We can take the example of acid-base titration. Suppose a solution of HCl is to be titrated against NaOH solution, the acid solution is taken in a beaker and a quinhydrone electrode or glass electrode is set up in it. The electrode is then coupled with a standard calomel electrode as shown in the figure.
NaOH solution is added to the beaker from the burette first in a larger amount say 0.5ml at a time and then in a smaller amount say 0.1ml or less near the endpoint.
After each addition, the emf of the cell is recorded. The emf is then plotted against the volume of the alkali added.
Potentiometric titration curve
The titration curve is obtained by plotting the emf of the cell against the volume of alkali added. The shape of the titration curve obtained in the case of strong acid and strong base is shown in the following figure.
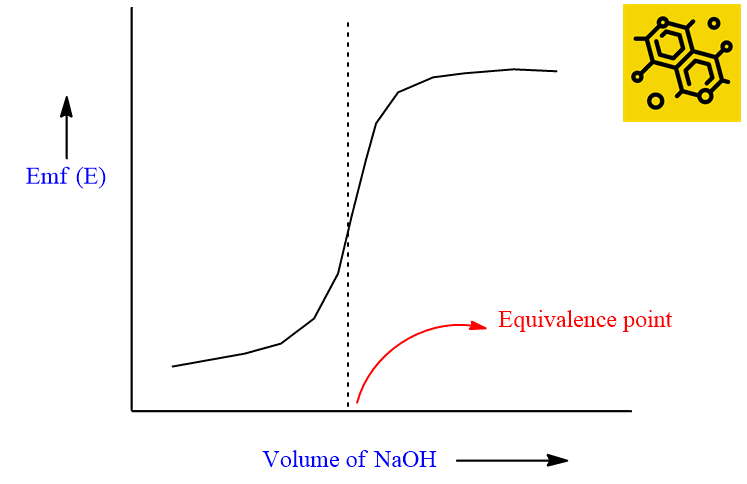
The steepest portion of the curve indicates the equivalence point. In case the solution is very dilute or if the acid and base involved are weak, the steepness of the curve may be less pronounced (less marked) and it is difficult to judge. In such a case, we can obtain an equivalence point from the first differential curve as shown below. The maximum of the curve indicates the equivalence point.
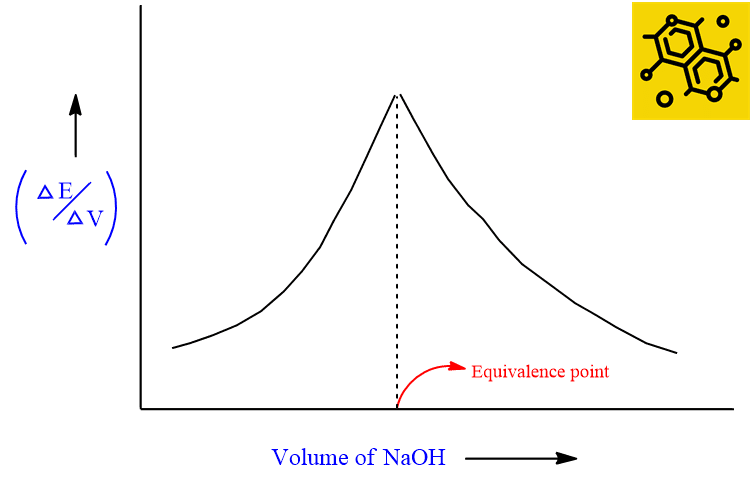
This P.T curve is known as the differential P.T curve. This curve gives an equivalent point more accurately than the curve obtained in the above case.
Advantages of potentiometric titration
- Potentiometric titration can be done between colored solutions as well where internal indicators are not useful.
- It can be done for the analysis of a mixture of two different acids with different dissociation constants using the same alkali solution.
- This titration can also be performed for the estimation of chloride (Cl–), Br-, and I– in a mixture containing the three components.
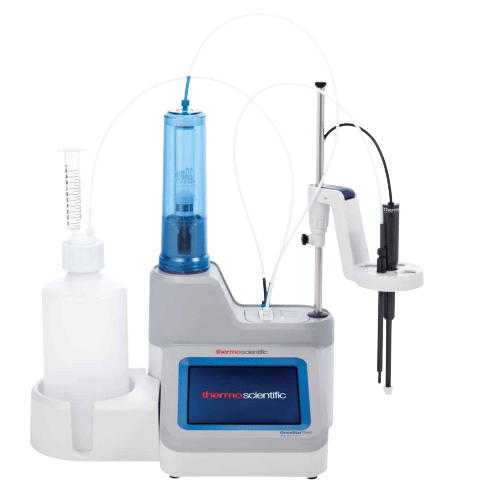
Potentiometric titrator
These titrators are automated titrators that are designed to make performing titration easier, reliable, and more reproducible in comparison to manual titration. Manual titrations take time and are sometimes imprecise owing to human error.







One Response
Thanks…share with your friend as well.