Table of Contents
ToggleThe word osmosis is derived from the Greek word osmos, which means to push. Thus, osmosis can be defined as the flow of the solvent molecules through a semipermeable membrane from a pure solvent to the solution or from a dilute solution to a concentrated solution. The flow of solvent molecules from the region of higher concentration to the region of lower concentration.
The hydrostatic pressure built upon the solution which just stops the osmosis of pure solvent into the solution through a semi-permeable membrane is called osmotic pressure.
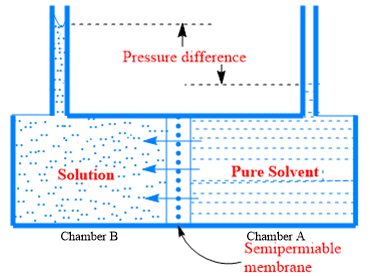
A solution is separated from the solvent by a semi-permeable membrane, which is permeable by the solvent molecules but not by solute. Since the vapor pressure and the chemical potential of the pure solvent are greater than these quantities in solution, the solvent molecules from chamber A will flow through the membrane into solution chamber B to equalize the chemical potential of the solvent in both chambers.
The chemical potential of solvent in chamber A is greater than its value in solution chamber B.
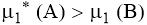
Van’t Hoff Factor
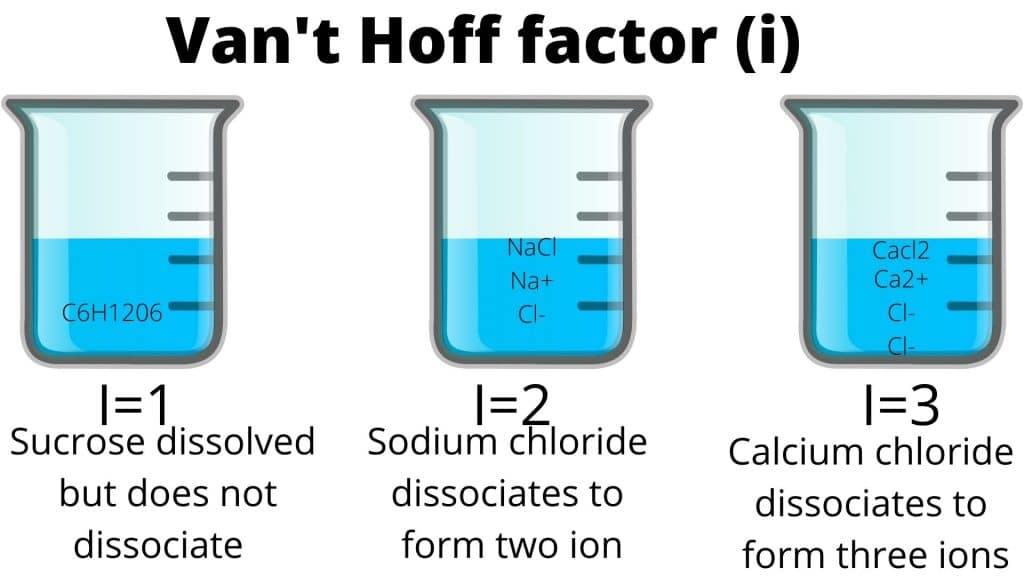
The Van’t Hoff factor is the ratio of the observed value of any colligative property to the normal value of the same colligative property if there is no association or dissociation. It is represented by (i).
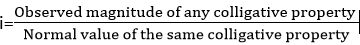
As the solute concentration increases the van’t Hoff factor decreases.For example, Vant Hoff’s factor for AlCl3
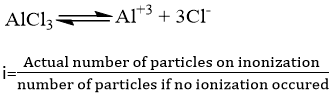
Suppose 1 mole of an electrolyte (AlCl3) is capable of forming v ions on complete dissociation. If the degree of dissociation be ‘α’ the total no. of particles in solution are:
no. of undissociated molecule = 1- α
no. of ions produced = αv
Total no. of ions produced = 1- α + αv
α is degree of dissociation
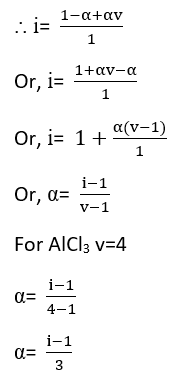
Van’t Hoff factor is related to the degree of dissociation of solution.
Van ’t Hoff Factors for 0.0500 M Aqueous Solutions of Selected Compounds at 25°C
| Compound | i(measured) | i(ideal) |
| Glucose | 1.0 | 1.0 |
| Sucrose | 1.0 | 1.0 |
| HCl | 1.0 | 2.0 |
| NaCl | 1.9 | 2.0 |
| MgCl2 | 2.7 | 3.0 |
| FeCl3 | 3.4 | 4.0 |
| AlCl3 | 3.2 | 2.0 |
The van’t Hoff factor is a measure of a deviation from ideal behavior. The lower the van ’t Hoff factor, the greater the deviation.
Isotonic Solution
When two solutions are separated by a semipermeable membrane and there is no flow of water across the membrane, the solution is said to be an isotonic solution. If the membrane is completely semipermeable, isotonic solutions are also iso-osmotic, i.e., they have the same osmotic pressure. Since osmotic pressure depends on the number of molecules, isotonic solutions have equimolar concentrations.
Different Between Hypertonic ani Hypotonic solution
| Hypotonic solution | Hypertonic solution |
| The two solutions are separated by a semipermeable membrane, the solution having lower osmotic pressure is called a hypotonic solution. | The two solutions are separated by a semipermeable membrane, the solution with higher osmotic pressure is called a hypotonic solution. |
| Hypotonic solutions have fewer solutes and more solvents. | Hypertonic solutions have more solutes and less solvent. |
| Hypotonic solutions cause the cell to swell because it promotes the shifting of water into it. | Hypertonic solutions cause the cell to shrink because it pulls the water out of the cell. |
| Hypotonic solutions can be used for dehydration and hypernatremia. | Hypertonic solutions can be used for cases of hemorrhage. |
FAQs
What is osmosis?
The flow of the solvent molecules through a semipermeable membrane from pure solvent to the solution or from a dilute solution to a concentrated solution is called Osmosis.
What is osmotic pressure?
The hydrostatic pressure built upon the solution which just stops the osmosis of pure solvent into the solution through a semi-permeable membrane is called osmotic
What is an isotonic solution?
When two solutions are separated by a semipermeable membrane and there is no flow of water across the membrane, the solution is said to be an isotonic solution.
Why equimolar solutions of urea and sodium chloride are not isotonic?
Osmotic pressure depends on the number of molecules or no. of ions. Among urea and NaCl, NaCl is a strong electrolyte and shows abnormal behavior due to the molecular dissociation phenomenon. So they are not isotonic.
What is the hypertonic solution?
The two solutions are separated by a semipermeable membrane, the solution having lower osmotic pressure is called a hypotonic solution.
What is the hypotonic solution?
The two solutions are separated by a semipermeable membrane, the solution with higher osmotic pressure is called a hypotonic solution.






