Table of Contents
ToggleCovalent Crystals also called atomic crystals are the molecular solids in which the same or different atoms are joined together by covalent bonding. Atoms of the same or different kinds with no/small changes in electronegativity form the structural units of such crystals. Unlike Ionic crystals, Covalent bonds are directional in nature. The continuous networks of atoms in the lattice form a connection that binds the entire crystal together as a single big molecule. The attraction between atoms in the crystal lattice takes place as
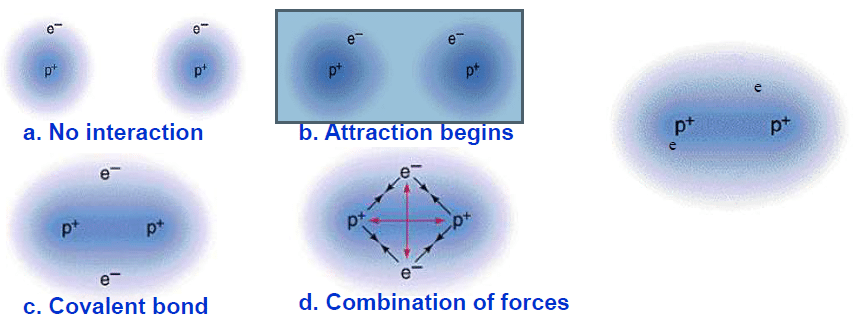
Examples of Covalent Crystal
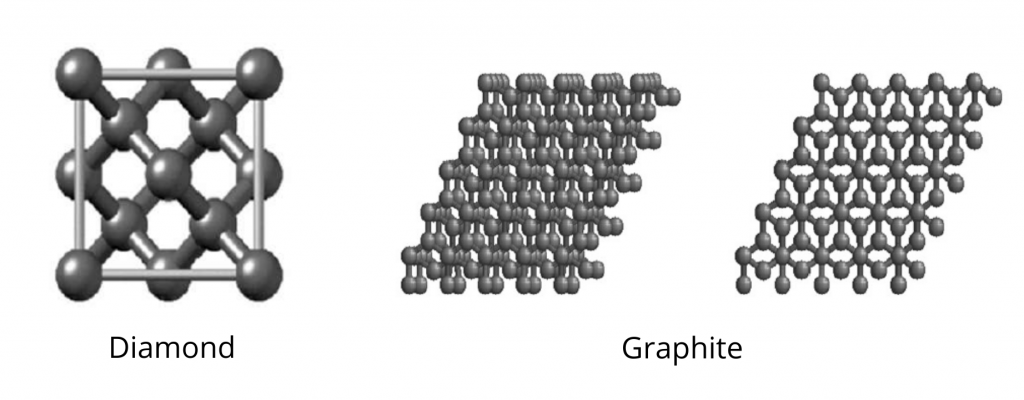
Some of the examples of covalent crystals are diamond, graphite, silicon dioxide, and so on.
Diamond
It is composed of carbon atoms that have been sp3 hybridized. Each carbon atom is connected to four other carbon atoms in a tetrahedral array to build a massive network of carbon rings with six members. In other words, the unit cell of the diamond is a face-centered cubic array of carbon atoms with four extra carbon atoms inserted into four tetrahedral holes. As the crystal is closed packed arranged in a three-dimensional lattice, it possesses a high density and refractive index and is, therefore, the hardest substance.
Graphite
It is made up of layers of fused six-membered rings of sp2 hybridized carbon atoms, with each carbon bound to three others, and atoms arranged in alternate layers of graphite. Every other carbon atom in one layer is directly under/above the center of a six-membered ring in an adjacent layer and hence forms the staggered layers.
Properties of Covalent Crystals
- Melting and Boiling point: As the atoms are held together by the covalent bonding via sharing of electron between atoms, it posseses strong forces, and hence high amounnt of energy is required to break this bond. Thus, Covalent crystals have high melting and boiling point.
- Hardness: Because of the directional properties of the covalent bonds, the atoms are strongly bounded in the lattice and cannot be easily displaced or compressed. This is the reason for the hardness of covalent crystals.
- Solubility: Covalent crystals are insoluble in polar solvent, but soluble in non-polar solvent.
- Conductivity: Since the electrons are tightly held by the atoms and cannot move through the lattice, covalent crystals possesses poor electrical conductivity.
- Brittleness: When a crystal is subjected to sufficient force, covalent bonds are broken as the lattice is distorted, and as a result of which shattering occurs in the crystal. Thus, covalent crytals like diamond are highly brittle in nature.
Covalent Crystal Video
FAQs/MCQs
What is a covalent crystal?
Covalent crystal is molecular solids formed by the covalent interaction between atoms.
Do covalent bonds form crystals
Yes, covalent bonds form crystals by the sharing of electrons between atoms.
Is graphite a covalent crystal?
Yes, graphite is a covalent crystal.
covalent crystal structure
Covalent crystals form closed-packed structures in a three-dimensional lattice.






