Table of Contents
ToggleIn normal chemical reactions, the product increases, the reactant decreases with time and the intermediate species remain constant until equilibrium is reached. However, there are certain reactions where the concentration of intermediate will vary periodically between two limits (minimum and maximum) either in time or in space are called oscillatory reactions.
Oscillating chemical reactions
The chemical reactions in which the concentration of intermediate species oscillates (vary periodically) with time are called oscillating chemical reactions. In such a reaction, properties like color, absorption, or average potential can change during the experiment with more or less periodical evolution. Due to this fact, some oscillating reactions are known as chemical clocks.
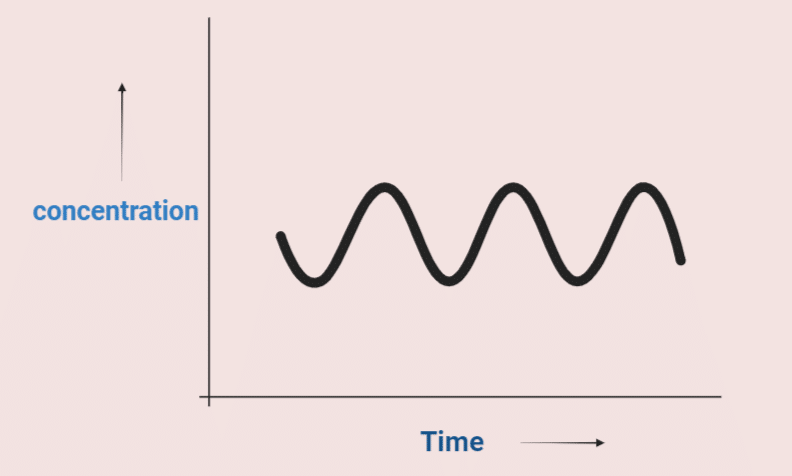
In an oscillating reaction, the product of one reaction becomes the reactant of another that regenerates the original reactant. Such a phenomenon is known as autocatalysis.
Criteria of Oscillating reaction
For a reaction to be an oscillating reaction in chemistry, it must fulfill the following criteria.
- The reaction system must be far from the equilibrium
- It must have at least two autocatalysis steps.
Examples of oscillation reactions
Some examples of oscillating reactions are listed below.
- Bray-Liebhafsky reaction (BL reaction)
- Briggs-Rauscher reaction (Oscillating Iodine clock)
- Belousov-Zhabotinskii reaction (BZ oscillating reaction)
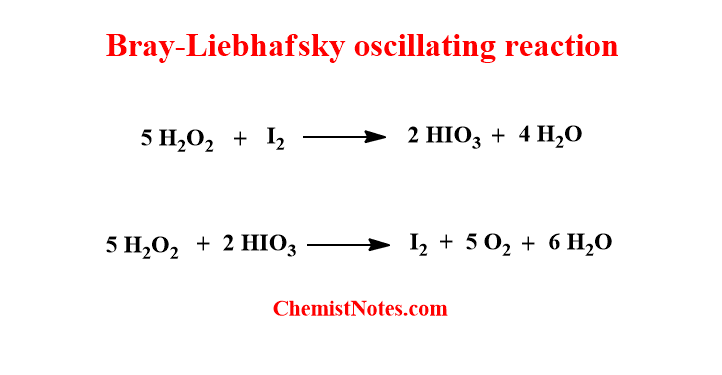
Bray-Liebhafsky oscillating reaction
The Bray-Liebhfsky reaction is a type of oscillating reaction which involves the decomposition of Hydrogen peroxide by iodate. This reaction is considered the first oscillating reaction reported by W.C Bray in 1921.
In this reaction, Hydrogen peroxide(H2O2) acts as oxidizing and reducing agent. In the first step, hydrogen peroxide oxidizes the iodine(I2) to iodic acid(HIO3). In the second step, it reduces the iodic acid back to iodine. In this way, the concentration of iodine seems to oscillate with time.
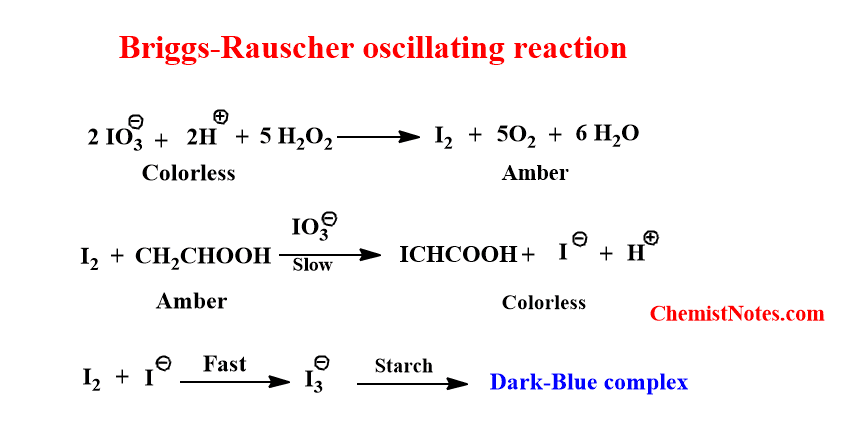
Briggs-Rauscher oscillating reaction
Briggs- Rauscher oscillating reaction is the chemical reaction in which the concentration of iodine and iodide oscillate with time, similar to the Bray-Liebhfsky reaction. This was first discovered by two scientists Briggs and Rauscher in 1973 and they identified this reaction as an iodine clock. In this reaction, the color is changed from colorless to amber to dark blue and that repeats with time. Thus, this reaction is also known as Briggs-Rauscher oscillating clock.
The Briggs-Rauscher oscillating reaction proceeds as:
- Initially, the iodide ion is reduced by hydrogen peroxide to iodine. In this process, the colorless solution changes to amber color.
- The generated iodine reacts with malonic acid to produce iodide ions. In this process the amber color change to colorless.
- The free iodine and iodide ion combine to form I3– which forms a dark blue complex with starch.
Belousov-Zhabotinskii oscillating reaction
The Belousov-Zhabotinskii reaction is another example of an oscillating reaction in which color (concentration) oscillates with time. This was first discovered by Belousov in 1959 during the Cerium-catalyzed oxidation of citric acid by bromate in aqueous sulfuric acid. Later on, in 1964, Zhabotinskii found a similar phenomenon when malonic acid is used instead of citric acid. Therefore, the Belousov-Zhabotinskii reaction involves the oxidation of malonic acid by bromate catalyzed by inorganic ions [ Ce(III)/ Ce(IV) ].
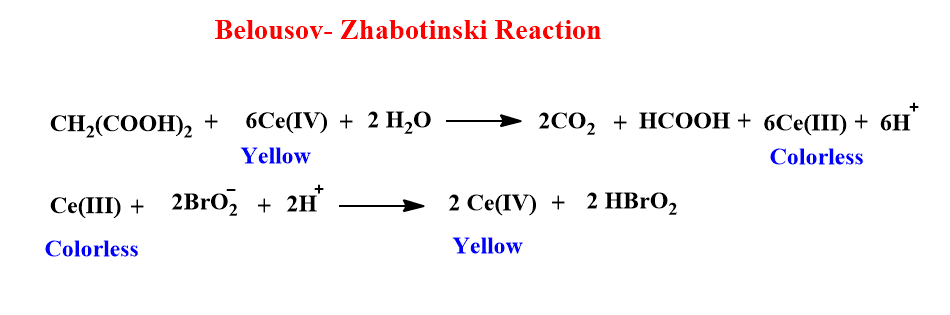
In the above reaction, ceric ion Ce(IV) (Yellow) is reduced in the first step while in the second step, cerous ion Ce(III) is oxidized to Ceric ion Ce(IV). Hence, the color of the reaction mixtures changes periodically from yellow to colorless while studying the BZ reaction due to the oscillation phenomenon in the reaction.
FAQs/MCQs
Do oscillating chemical reactions go to equilibrium?
Oscillating chemical reactions do not go to equilibrium because there is a periodic change in the concentration of reactant and product.