Table of Contents
ToggleDifferent liquids flow at different speeds. The resistance to the flow of liquid is called viscosity. The liquid which experiences high resistance in motion flows slowly and is said to be a more viscous liquid while the liquid which experiences low resistance in motion, flows rapidly and is said to be a less viscous liquid. Viscosity arises due to intermolecular forces of attraction between liquid molecules. The greater the intermolecular forces of attraction, the greater would be the viscosity.
Viscosity
Let’s consider the flow of liquid through a pipe in order to better understand the idea of viscosity. It is possible to imagine different concentric layers forming as liquid flows through a pipe. While the concentric layers in the center of the pipe move quickly, the concentric layers right next to the pipe’s wall move slowly. From the wall to the center of a tube, the velocities of several concentric liquid layers continue to rise. One can understand this principle by using a glass rod to rotate the water in a beaker.
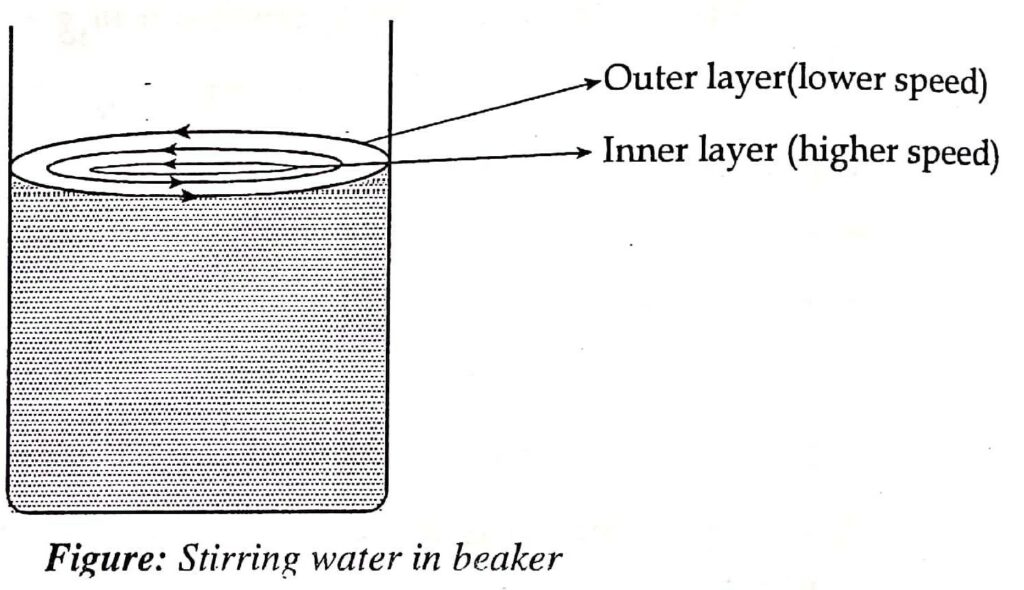
The velocities of several concentric layers steadily decrease as the stirring of the water is stopped. When this happens, the concentric layers of liquid are seen to move rapidly if the finger is put in the middle of the beaker rather than the side, where they are nearly stationary.
The following diagram helps to explain how liquid moves through a pipe at various speeds in a series of concentric layers. In the figure, the longer line denotes a higher velocity and the shorter line a lower velocity.
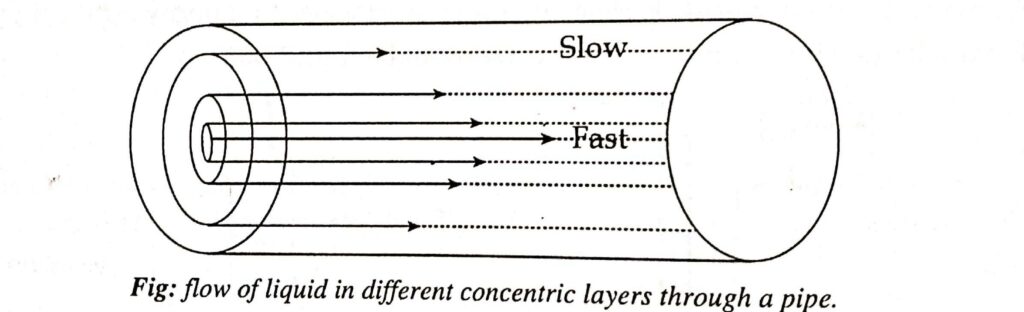
The liquid layer just adjacent to the wall of the tube is almost stationary and the velocity of each layer gradually increases towards the center. The liquid layer flowing through the center is the fastest.
In this case, the slow-moving liquid layer retards the speed of fast moving adjacent liquid layer. This retarding force is called viscous force and the property is viscosity. Let dx be the distance between two adjacent layers and dv be the difference in velocity of adjacent layers. Thus the velocity gradient is dv/dx.
According to Newton, the viscous force depends on the following factors:
- Directly proportional to the cross-sectional area i.e. F ∝ A
- Directly proportional to the velocity gradient between the concentric layers i.e. F ∝ (dv/dx)
Combining both these, we get
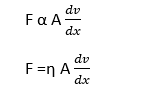
Where η is a proportionality constant and is called the coefficient of viscosity. It depends on the nature of the liquid.
Unit of Coefficient of Viscosity
In CGS system, the unit of coefficient of viscosity is dyne.sec.cm-2 which is also called poise.
In S.I. system, η = Netwon(N)/m2 = m/m.sec-1 = N sec m-2
Note: 1 decapoise = 10 poise
Fluidity (ϕ)
The reciprocal of viscosity is called fluidity. It is denoted by ϕ.
ϕ = 1 / η
Determination of Viscosity
Please click on this for the determination of viscosity of liquid by Ostwald’s viscometer method.
Effect of Temperature on Viscosity
When the temperature is increased, the kinetic energy of molecules is also increased and it weakens the intermolecular force of attraction. It decreases the coefficient of viscosity. Arrhenius and Guzman gave an empirical expression that shows the relation between temperature and coefficient of viscosity.
η = A eEa/RT
Where, η = coefficient of viscosity, A= constant, Ea = Activation energy, R = universal gas constant, and T = absolute temperature.
As temperature increases, the viscosity of a liquid decreases, whereas the viscosity of a gas increases i.e. viscosity of a liquid decreases with an increase in temperature, and the viscosity of gases increases with an increase in temperature.
The decrease in viscosity with an increase in temperature is true for those liquids whose molecules are not changed during heating. If molecular form undergoes change on heating, then the above generalization is ruled out. This is exemplified by liquid sulphur.
Application of Viscosity
- Applicable for the selection of lubricants for machinery use.
- Knowledge of the coefficient of viscosity may give an indication of the extent of a cohesive force, hydrogen bonding, and boiling point.
- At high fever conditions, the viscosity of blood decreases due to an increase in temperature.
- On the basis of blood viscosity, the condition of high blood pressure and strain on the heart can be explained. Due to viscosity, capillaries with arteriosclerosis (hardening of the arteries) have smaller capillary diameters and provide resistance to blood flow.
- Blood becomes more viscous and the corpuscles swell as the blood’s carbon monoxide concentrations increase.
Viscosity video
References
- Halliday, David; Resnick, Robert; Krane, Kenneth S. (2010-04-20). Physics, Volume 2. John Wiley & Sons. p. 342.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






