Table of Contents
ToggleReversible and irreversible cells are kinds of electrochemical cells in which the cell reaction can be reversed in the first one and cell reaction can not be reversed in the second one, by applying external emf. The basic criteria for cell reversibility, introduction, and examples have been discussed in this post.
Reversible and Irreversible cell
Reversible cell
A cell is said to be reversible if it satisfies the following three conditions:
- If an emf exactly equal to that of a cell is applied from an external source, the chemical reaction taking place in the cell will stop i.e no current flows.
- If the external emf is slightly greater than the actual emf of the cell applied, the current will begin to flow In the opposite direction and the cell reaction gets reversed.
- If the external emf is slightly lower than that of the actual emf of the cell applied to it, a very small amount of current will flow corresponding to a very small amount of chemical changes taking place in the cell.
This can be explained further in detail by using the following example:
Let us consider a Daniel cell as a reversible cell,
Zn|ZnSO4(1M)|| CuSO4(1M)|Cu (E=1.01 v)
When external emf equal to 1.01 v is applied in opposite direction, the cell reaction stops. When external emf is slightly greater than 1.01v the chemical reaction in the cell gets reversed i.e the current start to flow in opposite direction.
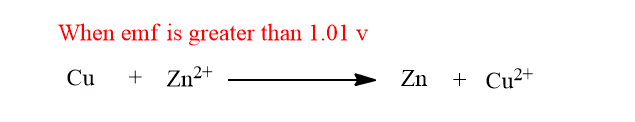
When external emf is slightly lower than 1.01v the chemical reaction doesn’t change but the extent of reaction is lowered and the flow of current is also lowered.
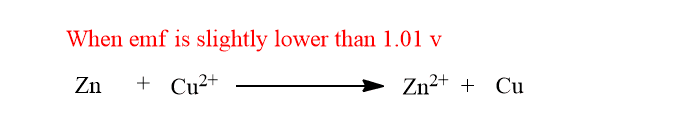
Irreversible cell
A cell is said to be irreversible if it does not satisfy the above criteria of reversibility. For example: Consider a cell consisting of Zn rod and Ag rod immersed in a dilute solution of H2SO4. When two rods are connected with wire, the following chemical reaction takes place.
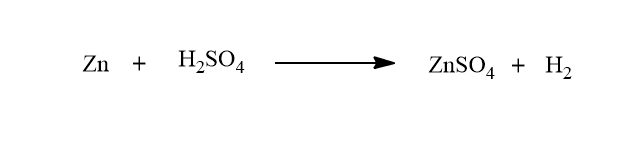
Here, Zn dissolves at one electrode, and H2 is evolved at another electrode.
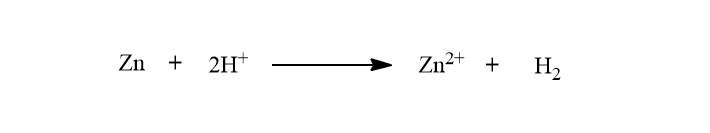
When external emf is greater then Ag dissolves at one electrode and H2 is evolved at another electrode.
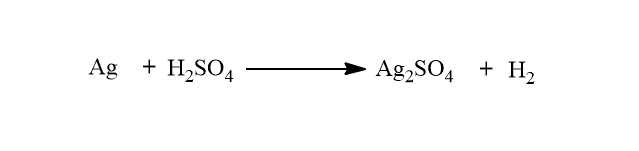
Thus, the condition of reversibility is not satisfied i.e chemical changes are not reversed. Hence, the above cell is not reversible but is irreversible.






