Table of Contents
ToggleDefine solubility product: The ionic solid substances dissolving in water are dissociated into cations and anions. If the concentration of the ions is increased, there is a high chance of collision between them and they collide with each other to form an original ionic compound. Therefore, there exists a dynamic equilibrium between solid ionic compounds and the ions, which is termed ionic equilibrium.
Define solubility product
Solubility product of sparingly soluble salt
Let us consider a sparingly soluble salt AB, the equilibrium can be shown as:
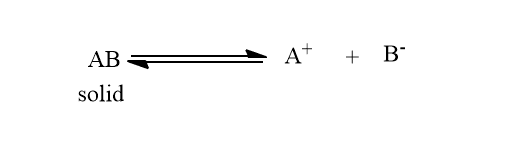
At saturated solution, the rates of dissociation and rate of recombination become exactly equal thus, a dynamic equilibrium exists. Therefore, the saturated solution can be defined as that solution that does not have the ability to dissolve more solute in the given solvent. Hence, the concentration of a substance in the saturated solution is termed the solubility of that substance.
As we know, AgCl is one of the sparingly soluble salt. The solubility product of Agcl is 1.56 ×10-10 mol2L-2and the equilibrium in its aqueous solution is shown as:
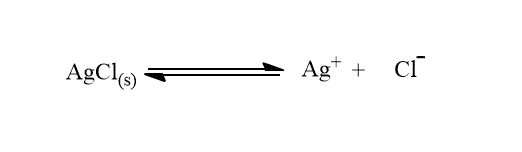
According to the law of mass action,
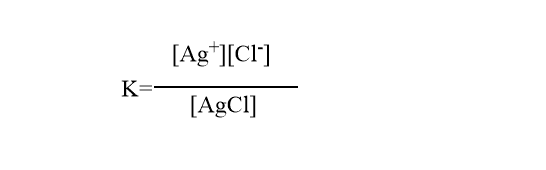
In a saturated solution, the amount of AgCl remains constant, therefore we can write [AgCl]=constant=x
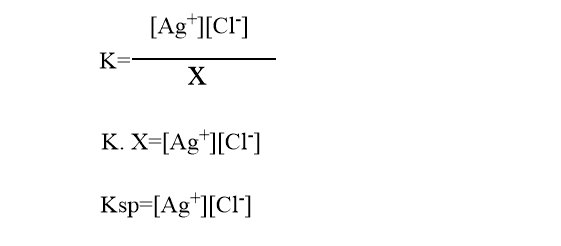
The constant Ksp is known as solubility product which is equal to the product of ionic concentration in a saturated solution. The value of the solubility product of any salt remains constant at a given temperature. This is called the solubility product principle.
In general, for salts type AxBy,
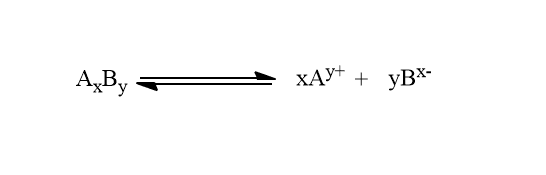
Solubility product=Ksp=[Ay+]x [Bx-]y
The solubility product of some sparingly soluble salts such as AgCl, AgBr, AgI, BaSO4, PbSO4 at 25oC is given below:
| Salt | Ksp value |
| AgCl | 1.56 ×10-10 |
| AgBr | 7.7 ×10-13 |
| AgI | 1.5× 10-16 |
| BaSO4 | 1.1 ×10-10 |
| PbSO4 | 1.06× 10-8 |
unit of solubility product
Units of concentration for solubility products are raised to the power of the stoichiometric coefficients of the ions in equilibrium. For example, AgCl’s solubility product has unit mol2L-2, PbCl2 solubility product has a unit mol3L-3.
Relation between solubility and solubility product
Let us consider a sparingly soluble electrolyte XY.
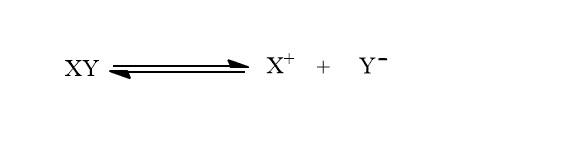
Let s mole/L be the solubility of XY then the concentration of X+ and Y– becomes s mole/L.
We know, Ksp= [X+][Y–]
= s×s= s2
s= (Ksp)1/2
This is the relationship between solubility and solubility product for binary sparingly soluble salts. We can calculate for other salts as well.
Condition of Precipitation
When the ionic product of salt becomes greater than the solubility product in the solution, due to an increase in the concentration of ions, the ions combine with oppositely charged ions to form a precipitate. Therefore, the ionic product plays a key role in precipitation. Whether the precipitation occurs or not depends on the value of the ionic product and solubility product value.
- If ionic product is equal to solubility product value, the solution is saturated and precipitation does not take place.
- If ionic product is greater than solubility product, solution is supersaturated and precipitation takes place.
- If the ionic product is less than solubility product, solution is unsaturated and precipitation does not take place.
Difference between ionic product and solubility product
The major difference between the ionic product and solubility product is that the ionic product is the product of ions in the solution either in saturated or unsaturated condition but the solubility product is the product of ions in the saturated solution. Other differences are listed below:
| Ionic product | Solubility product |
| Ionic product is the product of ions in either in saturated or unsaturated solution. | Solubility product is the product of ions only in a saturated solution. |
| It is applicable in any solution at any concentration. | It is applicable only in a saturated solution. |
| Its value changes with the concentration of the solution. | Its value remains constant at constant temperature for a given electrolyte. |
| If the ionic product of salt is greater than the solubility product, precipitation occurs. | If the solubility product is greater than the ionic product, precipitation does not occur. |
Application of solubility product
Some of the applications of solubility products are listed below:
- In determination of solubility of sparingly soluble salts.
- In determination of the condition of precipitaion of a salt.
- In qualitative analysis of salts, especially in selective precipitation of different group metal ions. Both concept of common ion effect and solubility product play important role in group separation of basic radicals.
Limitation of Solubility product principle
The solubility product principle is valid only for saturated solutions. If the concentration of ions is greater than 0.01 M, the Ksp does not remain constant, its value changes rapidly.






