Debye Huckel theory of strong electrolytes was put forward by Debye and Huckel in 1923 to explain a theory of interionic attraction in a dilute solution of an electrolyte, especially strong electrolytes.
Debye huckel theory of strong electrolytes
Arrhenius theory of ionization was limited to only weak electrolytes and did not explain the interionic attraction in dilute solution. Later, this was improved by Debye Huckel theory by considering the effects of interionic forces in dilute solution. The basic assumptions of Debye Huckel theory of strong electrolytes are given as:
- All strong electrolyte are almost completly ionized at dilution.
- There is no uniform distribution of cations and anions in the electrolytic solultion. But, the orientation of ions is such that one ion is surrounded by unlikes charges or oppositely charges.Thus each ion is surrounded by an ionic atmosphere of opposite charge. Solvent molecules are then attracted to this ionic atmosphere.
- The net charge on the ionic atmosphere is equal to but opposite to that on the central ion.
- The properties of electrolyte are determined by the interaction of central ion with its ionic atmosphere.
- The decrease in equivalent conductance with increase in concentration is due to decrease in ionic mobility because of greater interionic attraction.
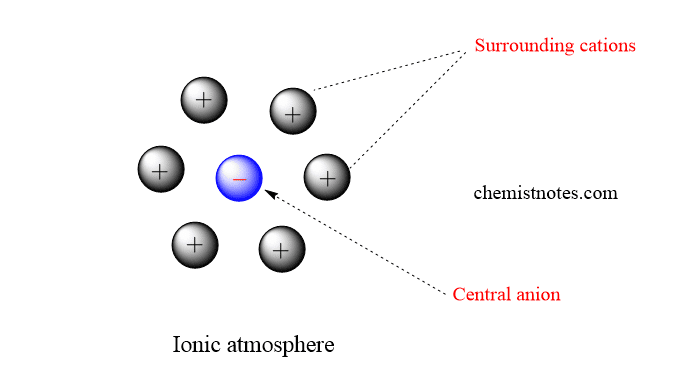
Debye-Huckel theory sought to explain the increase in the equivalent conductivity of the strong electrolyte during dilution, taking into account two facts: the asymmetric or mitigation effect and the electrophoresis effect.
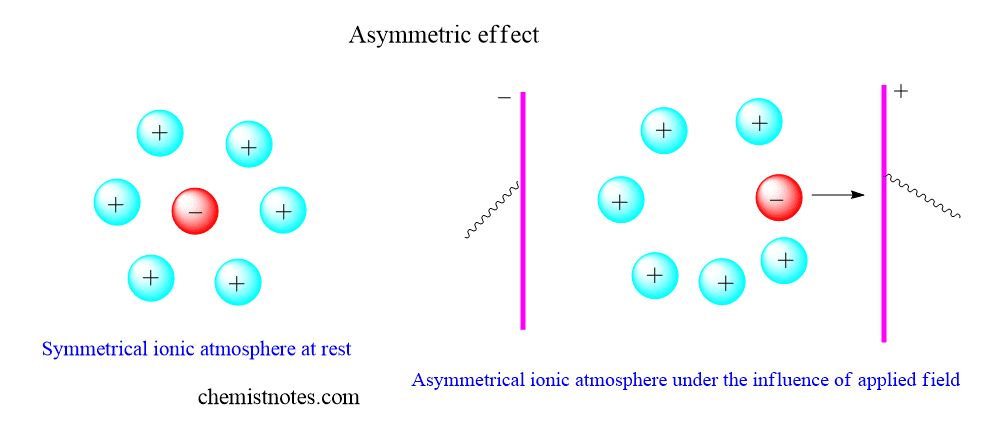
Asymmetric effect:
Due to the presence of coulombic interaction between ions, there is the formation of a well-organized group of structures called the ionic atmosphere. When an electric field is applied, cations start to move towards the cathode, and anions start to move towards the anode. If the ionic atmosphere contains cation as central ion and anions as surrounding ions, central cation tries to move towards cathode when an electric field is applied. Therefore, the cation tries to escape from the coulombic interaction of ionic interaction. So, the mobility of central cations decreases and the well-organized ionic atmosphere simply gets distorted. There is an asymmetry in the ionic atmosphere. Such an effect is called the asymmetric effect.
Electrophoretic effect:
In the solution, the solvent molecules are also attached to the ionic atmosphere. When an electric field is applied, the ionic atmosphere along with associated solvent molecules moves in the opposite direction to the movement of central ions along with the associated solvent. Therefore, the central negative ion moving towards the anode has to move in the way the ionic atmosphere with its associated solvent molecules which is moving in the opposite direction. Therefore, retarding force on the movement of ions arises due to solvent molecules. Such an effect is known as the electrophoretic effect.
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and CompanyLtd., New Delhi, 2012
- J. O’M. Bockris, A. K. N. Reddy and M. Gamboa-Aldeco, Modern Electrochemistry,Ionics, (2nd Edition), Vol. 1, Kluwer Academic/PlenumPublishers, New York, 1998






