Table of Contents
ToggleConductometric titration is a type of titration in which the endpoint of a titration is determined by measuring the conductance of the mixture. The conductometric titrations are based on the principle that the conductance of the solution depends on the number as well as the mobility of ions.
Conductometric titration definition
Conductometric titration is an electrical measurement method that determines the concentration of an electrolyte by measuring conductance at various stages of titration with another standard electrolyte. In this titration, the variation of the conductance of the solution with the addition of titrant is measured.
principle of conductometric titration
Let’s describe the principle involved in conductometric titration.
When an electrolytic solution (titrant solution) is added from a burette to another electrolytic solution, the conductivity of the solution changes when an ionic reaction occurs between these solutions.When one ion replaces another, the conductivity of the solution changes. If high-mobility ions like hydrogen ions are replaced by lower-mobility ions like sodium ions, the conductance of the solution decreases as we know the conductance of a solution depends on the number of ions and mobility of ions.
Similarly, if low-mobility ions are replaced by high-mobility ions in the solution during the course of the reaction, the conductance of the solution increases. The basic principle of conductometry or conductivity titration is based on the fact that ions of one mobility are replaced by ions of another mobility, thus the conductance of the solution changes during the course of the reaction.
The conductometric titration curve is obtained by plotting graphically the conductance against the volume of solution(titrant) added from the burette. The point of break or intersection point in the conductometric titration curve gives the endpoint of the titration.
The most important note observed during titration is that the volume of the solution should not change significantly during the addition of the titrant. Therefore, the titrant should be at least 10 times the concentration of the solution to be titrated in order to keep the volume change small. If it is not, you need to correct the reading by using the following relation.
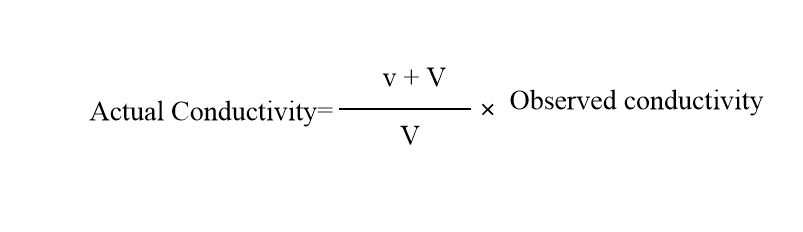
.
Types of conductometric titration
Conductometric titration of strong acid vs strong base( HCl vs NaOH)
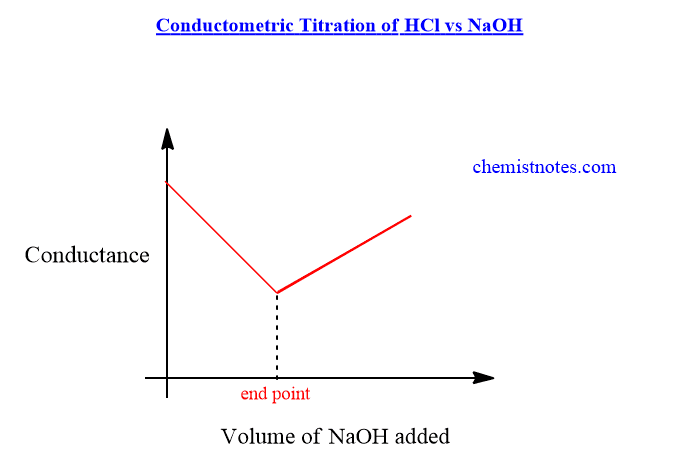
When a strong acid such as HCl taken in a beaker is titrated with a strong base such as NaOH, the fast-moving hydrogen ions are replaced by slow-moving sodium ions and the conductance decreases gradually up to the equivalent point. Let’s see the reaction.
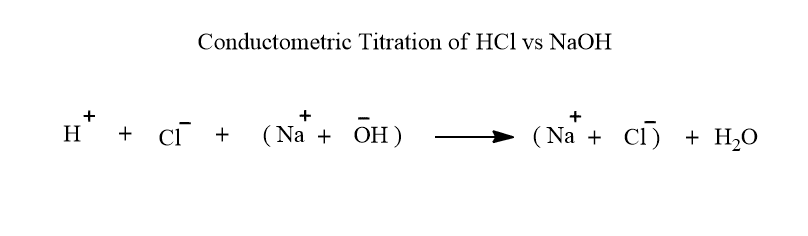
After the endpoint, On adding NaOH solution librates fast-moving OH- ions to the solution, and hence the conductance increases gradually increases. The conductometric titration curve or the graph of HCl vs NaOH is shown below.
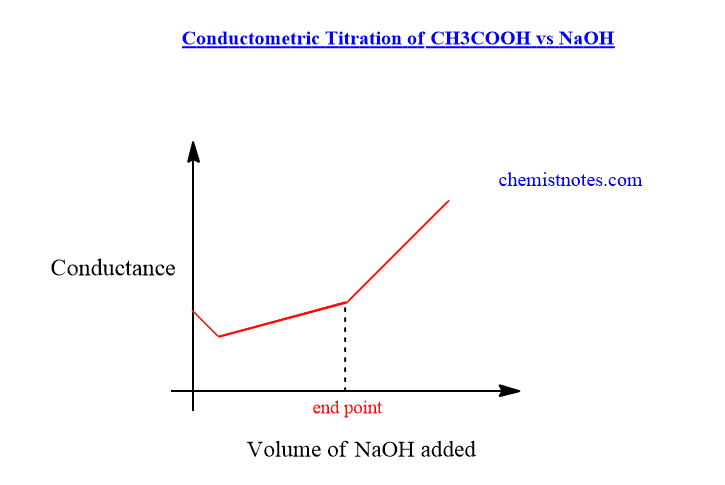
Conductometric titration of weak acid and strong base(CH3COOH Vs NaOH)
Initially, the conductance of acetic acid solution is low because it is a weak acid and weakly dissociated. When we add NaOH solution from a burette, there is a slight decrease in the conductance of the solution which is due to the substitution of free H+ ions by slow-moving Na+ ions. On further addition of NaOH solution, the conductance of solution increases slightly due to the formation of highly ionized compound CH3COONa, till endpoint.
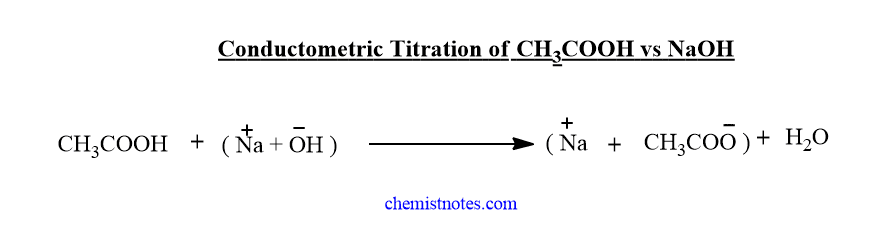
After the endpoint, the addition of NaOH further, there is an increase in the conductances of the solution sharply which is due to free OH–ions. The conductometric titration graph of CH3COOH vs NaOH is shown below.
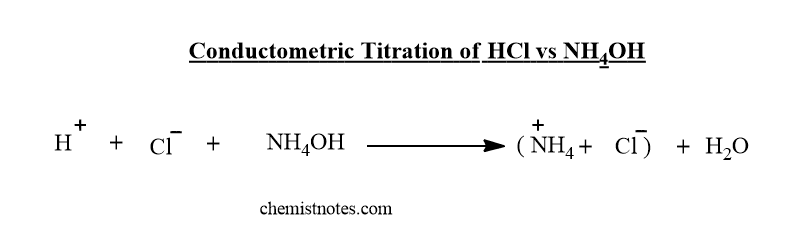
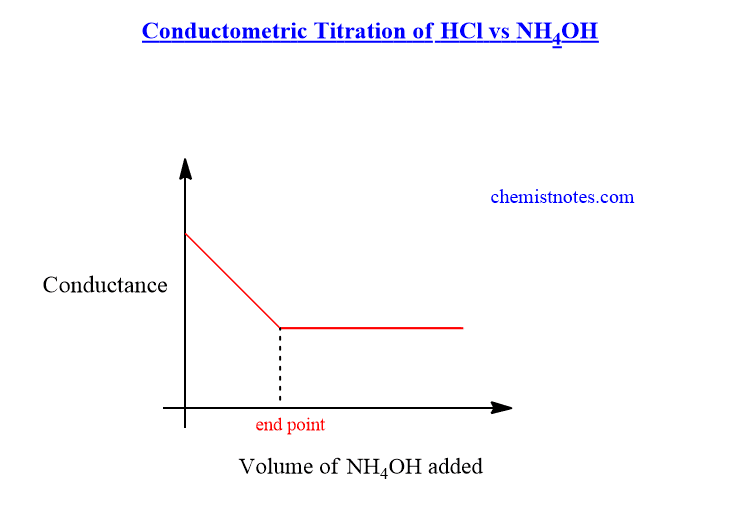
Conductometric titration of strong acid and weak base( HCl vs NH4OH)
In the beginning, the conductance of the HCl solution is very high due to the liberation of fast-moving H+ ions. But, when a weak base such as NH4OH is added from the burette, the fast-moving H+ ions are replaced by slow-moving NH4+ ions. Therefore, the conductance decreases until the endpoint is reached.
After the endpoint, when further NH4OH is added to the solution, the conductance of the solution does not change because NH4OH is a weak base. The conductometric titration graph of HCl vs NH4OH is shown below.
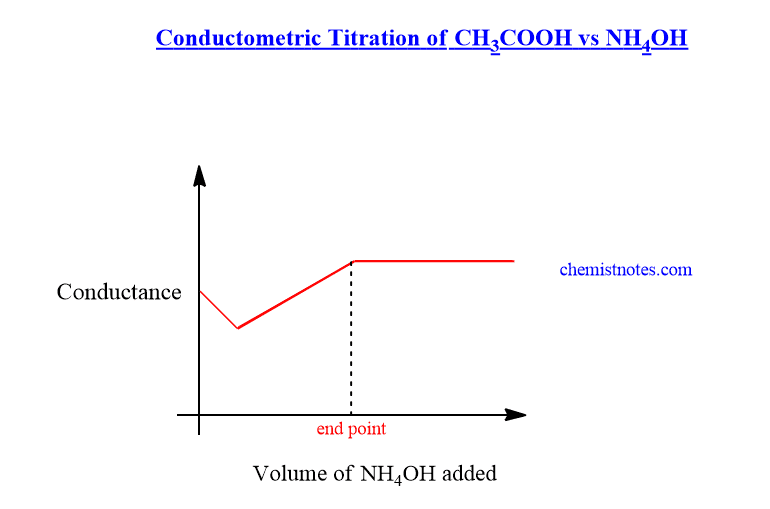
Conductometric titration of weak acid and weak base( CH3COOH vs NH4OH)
In the beginning, the conductance of weak acid solution is very low due to low ionization. But when NH4OH is added gradually to the acid solution, the conductance slightly decreases due to the substitution of H+ ions by slow-moving NH4+ions. On further addition of NH4OH solution, the conductance of solution increases til the endpoint due to the formation of highly ionized salt.
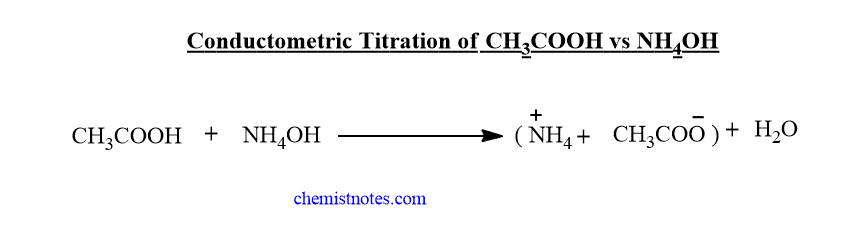
After the endpoint, the further addition of NH4OH solution does not cause to increase in conductance due to a very small extent of ionization. The conductometric titration graph of CH3COOH vs NH4OH is shown below:
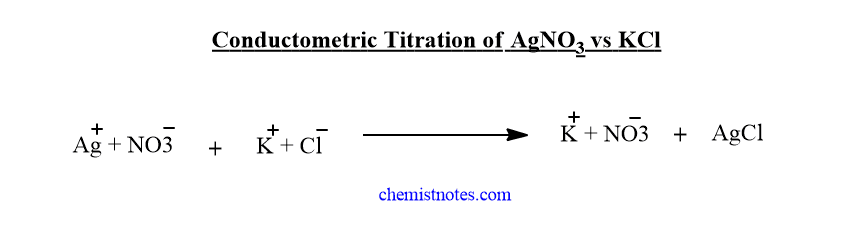
Conductometric precipitation titration( AgNO3 vs KCl)
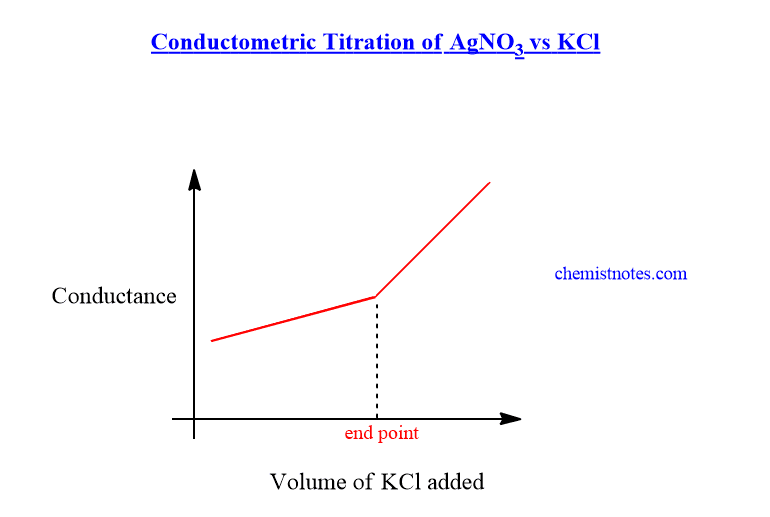
In the beginning, the AgNO3 solution has a certain value of conductance. But when the KCl solution is added to the AgNO3 solution, Ag+ ions are substituted by K+ ions. It means slow-moving Ag+ ions are replaced by fast-moving K+ ions. In another word, the ionic mobility of potassium ions is greater than that of silver ions. Therefore, there is a gradual increase in the conductance of the solution till the endpoint.
After the endpoint, further addition of KCl produces more K+ ions and Cl– ions, therefore, the conductance of the solution increases sharply. The conductometric titration curve of the AgNO3 vs KCl solution is shown below.
Advantages of conductometric titration
Some of the main advantages of such titration are given:
- It is quite difficult to select suitable indicators for the titration of colored solutions. In such cases, conductometric titration can be carried out.
- Titration of very diluted solution and weak acids versus weak bases can not be carried out in volumetric titration but titration of such acid bases can be carried out quite easily with the help of conductometric titration.
- In a volumetric titration, special attention is needed near the endpoint to detect the color change but in this titration, no such special care is required near the endpoint.
FAQs/MCQs:
What is conductometric titration?
Conductometric titration is a type of titration in which the endpoint of a titration is determined by measuring the conductance of the mixture.
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and CompanyLtd., New Delhi, 2012






