Table of Contents
ToggleCyclic voltammetry (CV) is one of the most adaptable electroanalytical methods for analyzing electroactive substances. Its main applications include figuring out formal redox potentials, spotting chemical processes that occur before or after electrochemical reactions, and assessing electron transfer kinetics.
Principle of Cyclic Voltammetry
A periodic, triangular potential excitation signal is used in cyclic voltammetry to cycle an electrode’s potential between two limits while measuring the resulting current, as shown below.
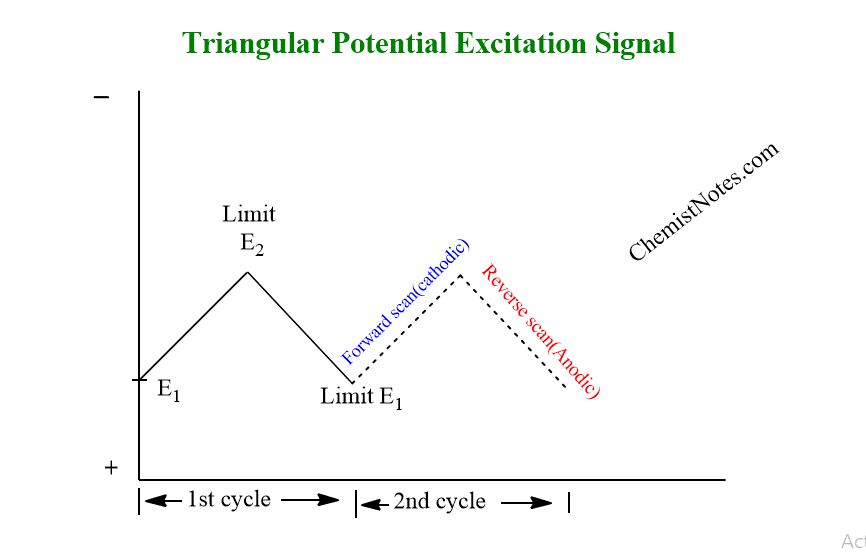
The electrode potential is linearly increased to either a more negative or positive potential, and then it is linearly decreased to return to the initial voltage.
Any analytes that can be reduced via the potential scan’s range create a current peak during the forward scan. The current will rise as the potential approaches the analytes’ reduction potential, but it will then start to decline as the concentration of the analytes at the electrode surface decreases.
As the applied potential is reversed, the product created in the initial reduction process will be reoxidized, producing a current with reverse polarity from the forward scan. This oxidation peak will usually have a similar shape to the reduction peak.
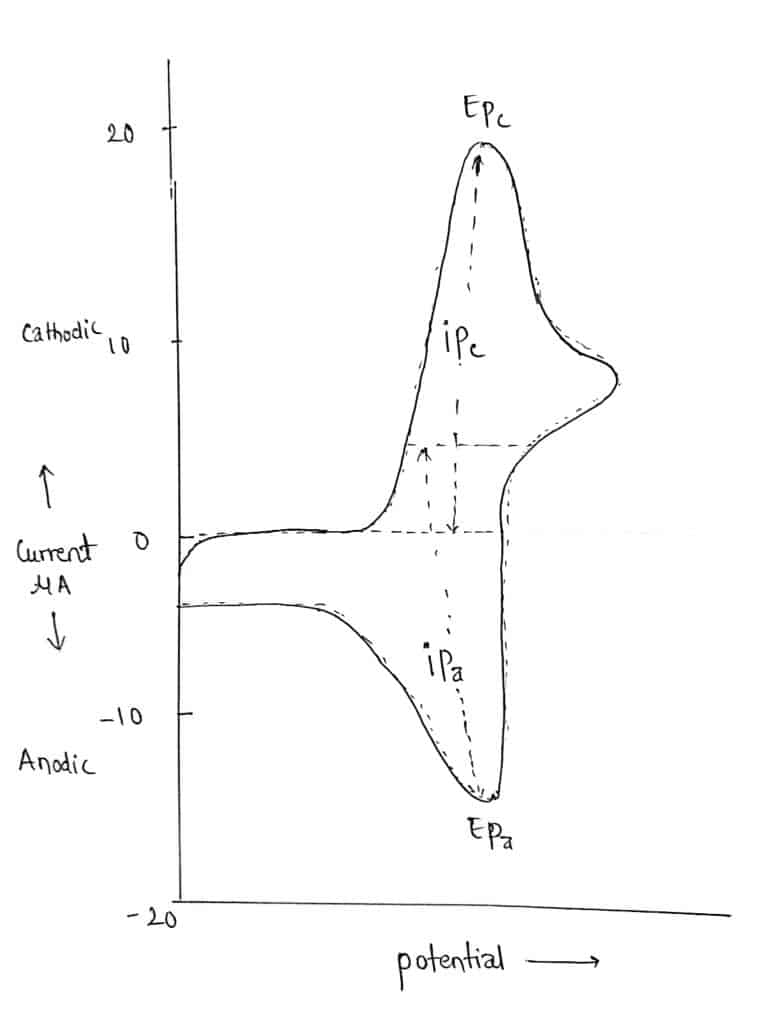
Steps in Cyclic voltammetry
First of all, choose a starting potential where only charging current flows. After choosing the appropriate starting potential, scan the potential either in +ve or –ve direction to a limit i.e certain fixed value. Then resulting current is measured. At last, reverse the direction of the potential from the limit back to the initial potential limit and the resulting current is noted.







2 Responses
Amperometry is not loading .
There is problem check that and upload.
Thank you for your information. Sure, we gonna check and update it soon.