Basic principle of voltammetry can only be discussed after the general introduction of voltammetry. Voltammetry is a group of electroanalytical methods in which information about the analytes is obtained by measuring the current as a function of applied potential. A variable potential difference is applied between a reference electrode (e.g. Ag/AgCl) and an indicator electrode, referred to as the working electrode, in voltammetric techniques. The basic principle of voltammetry has been discussed in this post.
Basic principle of voltammetry
When the voltage at the working electrode reaches a point where a species in the solution under investigation is either oxidized or reduced. For reversible electrochemical reaction, a reaction so fast that equilibrium is always re-established as changes are made, which can be described by
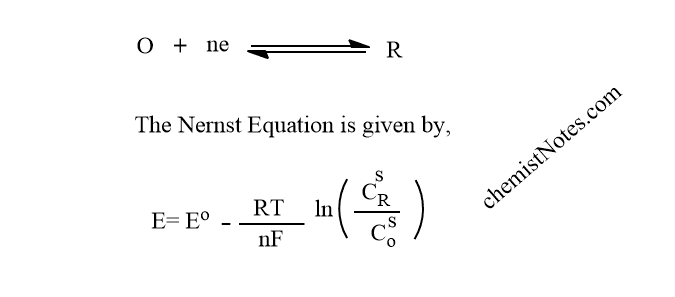
When a voltage E is supplied, it causes the concentrations of O and R at the electrode surfaces to be forced into a ratio according to the Nernst equation. When the voltage provided to the electrode is altered, the CR/Co ratio at the surface changes, satisfying the equation above.
The ratio CR/Co increases as potential are made more negative, i.e. O is lowered. In the same way, If the potential is made more positive, the ratio becomes smaller i.e R is oxidized.
We know that the current flows depend on the flux of materials to the electrode surface. When additional O or R is formed at the surface, the higher concentration acts as a driving force for their diffusion into the solution’s bulk. Similarly, when O or R is destroyed, the lower concentration encourages new materials to diffuse from the bulk solution due to concentration differences.
The resulting concentration gradient and mass transport are described by Fick’s law, which states the flux of matter (Φ ) is directly proportional to the concentration gradient.
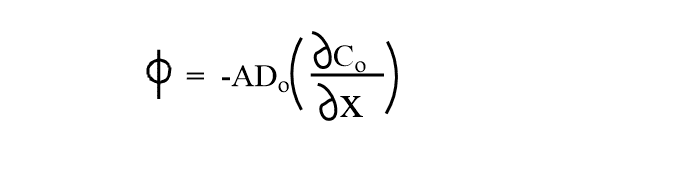
Where Do is the diffusion coefficient of O and x is the distance from the element’s surface.
The flux of O or R at the electrode surface controls the rate of reaction and thus the faradaic current flowing in the cell. The current is a measurement of how quickly a substance is reduced or oxidized at the electrode surface. Many other parameters influence the actual value of this current, including the redox species concentration, electrode size, shape, materials, solution resistance, cell volume, and the number of electrons transported.
In addition to diffusion, mass transport can also occur by migration or convection. The movement of a charged ion in the presence of an electric field is referred to as migration. The use of a supporting electrolyte at a concentration 100 times that of the species being tested removes the influence of migration current in voltammetry.
Convection is the measurement of the electroactive species by thermal currents, by density gradient present in the solution, or by stirring the solution or rotating the electrode. Convection must be avoided or precisely regulated in order to enable controlled transport of analyte to the electrode through diffusion.
References
- Vogel’s Textbook of Quantitative Inorganic Analysis, (4th Edition), ELBS/Longman
Scientific & Technical, London, 1978 - D. A. Skoog, D. M. West, F. J. Holler, and S. R. Crouch, Fundamentals of Analytical Chemistry, (8th Edition), International Student Edition, Books/Cole Cengage Learning, Belmont, USA, 2004.






