Table of Contents
ToggleCommon ion effect is simply the effect observed due to the presence of a common ion. The Common ion effect is an effect in which the degree of dissociation of a salt (weak electrolytes) is reduced by the addition of a common ion. We discuss its examples and applications in detail.
What is common ion effect
Weak acids or weak bases are dissociated in small fractions in water. The dissociated ions are in equilibrium with undissociated molecules. Let AB be an electrolyte that dissociates in water as:
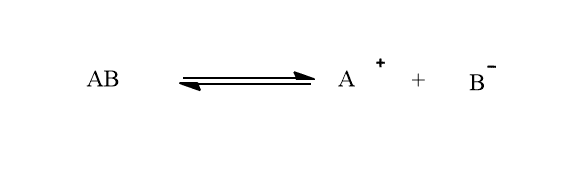
There exist an equilibrium between AB and A+ and B- ions which can be termed as ionic equilibrium.
If another salt or strong electrolyte having a common ion to electrolyte AB is added to the solution of AB electrolyte, the degree of ionization of AB electrolyte( weak electrolyte) decreases. This can be explained as:
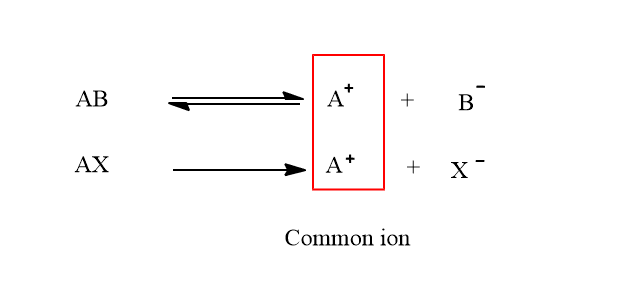
When strong electrolyte AX is added to the solution of AB, the concentration of A+ ions in the solution increases. According to Le chatelier’s principle, the equilibrium will shift to the left, hence decreasing the concentration of A+ions. It means the degree of ionization is suppressed.
Therefore, suppression of degree of ionization of weak electrolytes by addition of strong electrolyte having a common ion is called common ion effect.
Common ion effect example
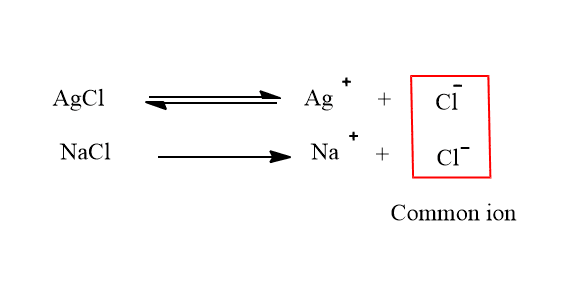
i. Suppression of solubility of AgCl by addition of NaCl
The ionic equilibrium of AgCl in its saturated solution can be shown as:
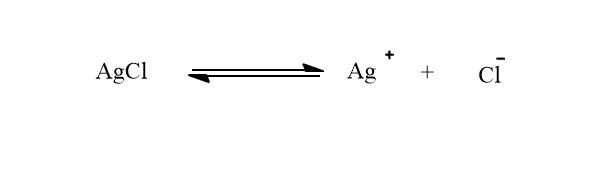
When NaCl is added to the solution of AgCl, the concentration of Cl- ions in the solution will increase.
Due to an increase in the concentration of Cl- ion, the equilibrium will shift towards the left to form more solid AgCl. Therefore, the solubility of AgCl is suppressed.
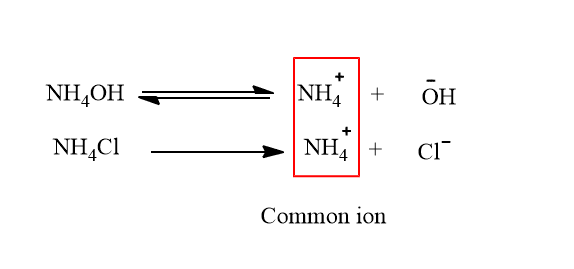
ii. Suppression of degree of ionization of NH4OH
When NH4Cl is added to the solution of NH4OH, the equilibrium shift to the left due to common ion NH4+.
Application of common ion effect
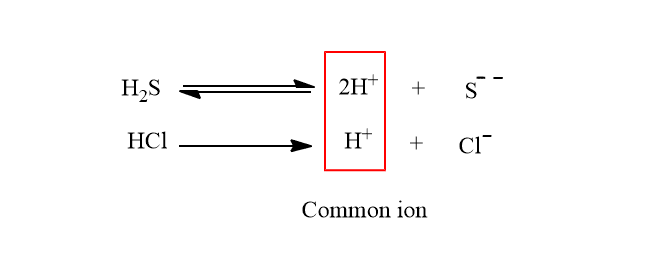
The concept of the common ion effect has a very important application in the qualitative analysis of salt. Let us explain the application by taking the example of group II metal ions ( Hg++, Pb++, Bi+++, Cu++, etc.) These are precipitated as their sulfides. The solubility products of their sulfides are very low and even small concentrations of sulfide ions can precipitate these metal ions. In such a case, the ionic product must be greater than the solubility product.
If a high concentration of sulfides is present in the solution, the metal ions of group IV will also get precipitated along with Group II metal ions. Therefore, for the reduction of S– ions concentration, H2S gas is passed through the solution of the salts in presence of dil. HCl, which suppresses the degree of ionization of H2S due to the common ion effect.