Table of Contents
ToggleAmperometric titration is the application of amperometry, which involves the measurement of the current produced by the electrochemical oxidation or reduction of an electroactive species, and is the foundation of amperometry. Thus, amperometry is the measurement of currents at constant voltage supplied to the dropping mercury electrode. The principle, advantages, and disadvantages of amperometric titration have been discussed in this post.
Amperometric titration
A titration in which the endpoint is determined by the current at a suitable applied potential across two electrodes as a function of the volume of the titrating solution is called amperometric titration. In another word, the phrase “amperometric titration” describes a group of titrations in which the equivalence point is established by measuring the electric current generated by the titration process.
Principle of amperometric titration
We know the limiting current is independent of the applied voltage impressed upon a dropping mercury electrode or working electrode. The only factor that affects the limiting current is the rate of diffusion of electroactive material from the bulk of the solution to the electrode surface if the migration current is almost eliminated by the addition of sufficient supporting electrolytes.
Hence, the diffusion current (limiting current-residual current) is proportional to the concentration of the electroactive material in the solution. If the residual and migration current is eliminated, the actual value of the limiting current almost depends on the diffusion current.
If some of the electroactive species are removed by interaction with the reagent, the diffusion current will decrease. This is the fundamental principle of amperometric titration or amperometry.
The observed diffusion current at a suitable applied voltage (constant voltage) is measured as a function of the volume of titrating solution. The endpoint is the point of intersection of two lines giving the change of current before and after the equivalence point.
Examples of Amperometry
This can be better understood by observing some examples.
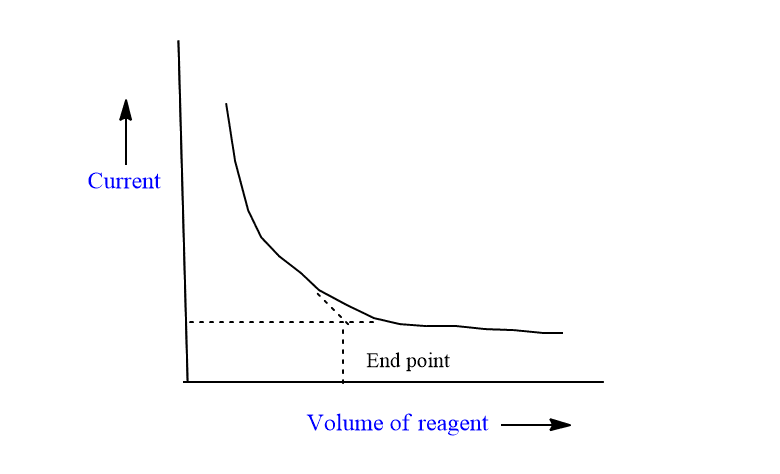
Titration of electroactive solute and inactive reagent
The titration of Pb++ ions vs SO4— is an example of this type of titration.
In this case, limiting current is due to lead ion and is maximum at the beginning. As the titrant (SO4—) is added, a precipitate of PbSO4 is formed and the limiting current decreases linearly with a decrease in lead ion. i.e with an increase in SO4— ions.
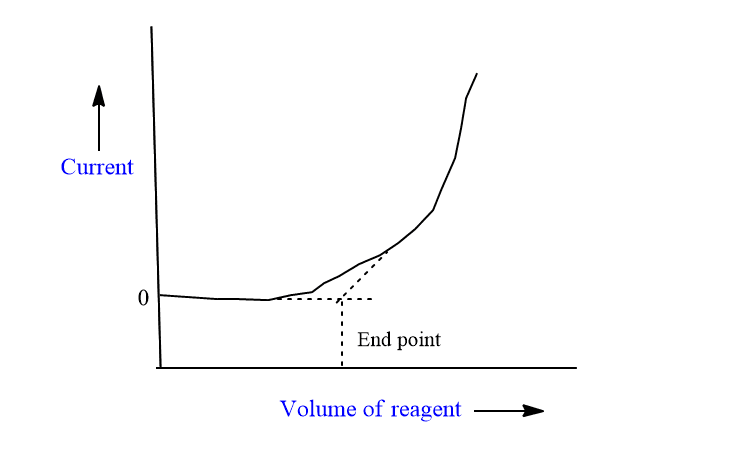
Titration of inactive solute and active reagent
The titration of SO4—vs Pb++ ion is an example of this type of titration.
In this case, the initial limiting current is almost zero and it remains minimum till the end point is achieved. The almost zero value of limiting current before the end point is due to electro-inactive SO4—ions. After the endpoint, the addition of Pb++ ions remains in the solution as ions, and the limiting current increases linearly with Pb++ concentration.
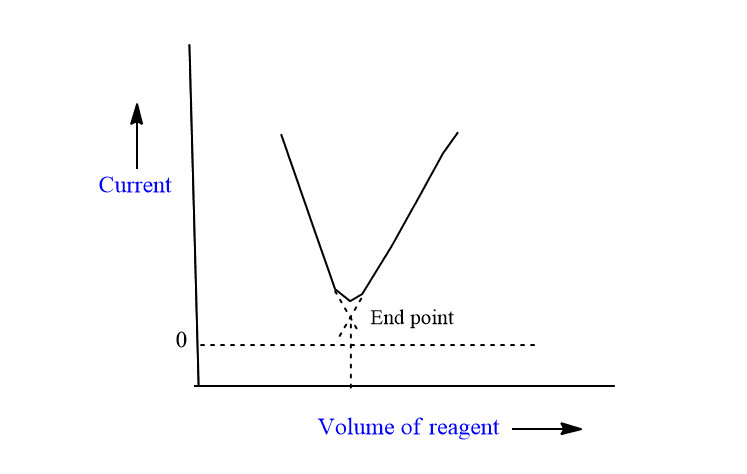
Titration of active solute and active reagent
The titration of Pb++ ion vs Cr2O7—ions is an example of this type of titration. In this case, initially high limiting current value is due to the electroactive Pb++ ion. As the Cr2O7— is added, it forms the complex, and the concentration of Pb++ ion decreases, thus the limiting current also decreases linearly with the addition of Cr2O7—till the endpoint. After the endpoint, due to the presence of electromotive-free Cr2O7—, the current again increases linearly. This results in a V-shaped curve.
Advantages of Amperometric titration
- It can be carried out rapidly since the endpoint is found graphically. A few current measurements at constant applied voltage before and after the endpoint is sufficient.
- It can be carried out for the determination of both organic and inorganic compounds, detection is based on the formation of precipitates and complex, redox reaction, acid-base reaction.
- It is applicable when visual or potentiometric methods are not possible.
- It shows high sensitivity and high precision.
- The depolarising substance which can’t be estimated polarographically, can be successfully estimated by using this titration
- It can be carried out in a dilute solution.
- The amperometric method is more accurate and sensitive as compared to polarography.
- As the current obtained in the case of amperometric titration is 20 times more than polarography. Therefore, a small concentration or trace of reducible species can be determined accurately.
- Several non-reducible substances like Mg++, PO4—, SO4—, etc can be determined by titrating them with reducible titrants.
Disadvantages of Amperometric method
The major disadvantage of the amperometric method is that sometimes co-precipitation gives inaccurate results. Moreover, the titration can’t be carried out at a potential more negative than -2 volt because hydrogen will be evolved.
Amperometric titration youtube video
References
- D. A. Skoog, Principle of Instrumental Analysis, (3rd Edition), Saunders College
Publishing, 1985. - Vogel’s Textbook of Quantitative Inorganic Analysis, (4th Edition), ELBS/Longman Scientific & Technical, London, 1978






