Table of Contents
ToggleBritish scientist Alexander Williamson first demonstrated ether synthesis in 1850 by using a new route. Hence, this reaction was named ” Williamson ether synthesis”. This is one of the suitable methods of preparing symmetrical as well as unsymmetrical ethers.
Williamson ether synthesis
Williamson ether synthesis is a two-step process of ether synthesis. This reaction involves the nucleophilic substitution reaction between an alkali alkoxide and alkyl halide forming ethers. This reaction is also known as Williamson etherification
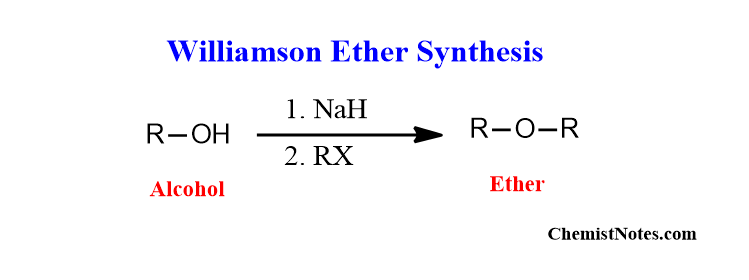
Alcohol is first deprotonated using sodium metal or sodium hydride or sometimes sodium hydroxide to generate an alkoxide ion. The formed alkoxide reacts with alkyl halide by a bimolecular nucleophilic substitution mechanism.
Williamson ether synthesis mechanism
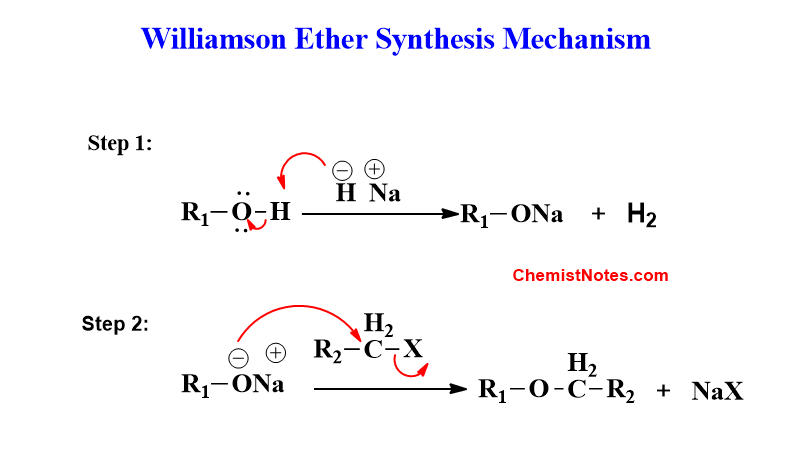
The mechanism of Williamson etherification completes in two steps.
Step 1: A hydride ion acts as a base and deprotonates the alcohol in the first step.
Step 2: The created alkoxide ion subsequently acts as a nucleophile and acts in an SN2 reaction with an alkyl halide.
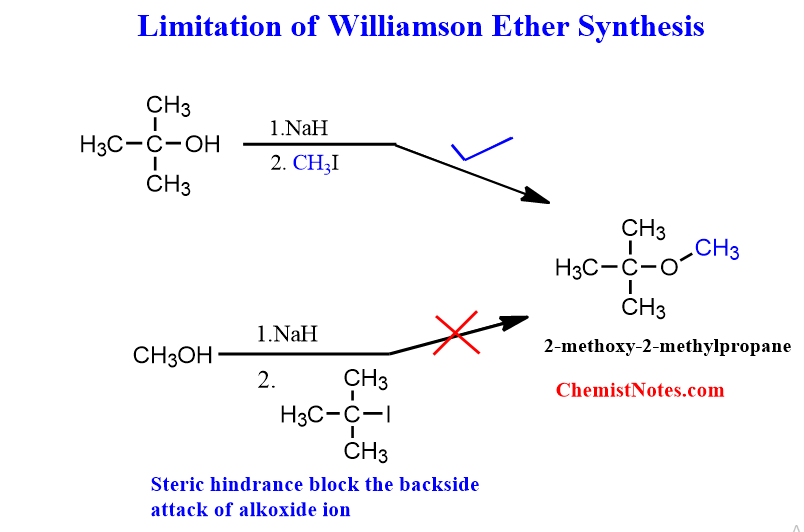
limitations of Williamson ether synthesis
The major limitation of Williamson ether synthesis is that bulky ethers can not be prepared by using secondary or tertiary alkyl halide. When methyl or primary alkyl halide is used, this method delivers the best result. But, If secondary or tertiary alkyl halide is used, the steric hindrance prevents the alkoxide’s backside attack. Moreover, these are less efficient because elimination is favored over substitution.
Example: di-tert butyl ether cannot be prepared by Williamson synthesis.
Let’s take another example of the synthesis of Tert-butyl-methyl ether to verify the above statements.
Some examples
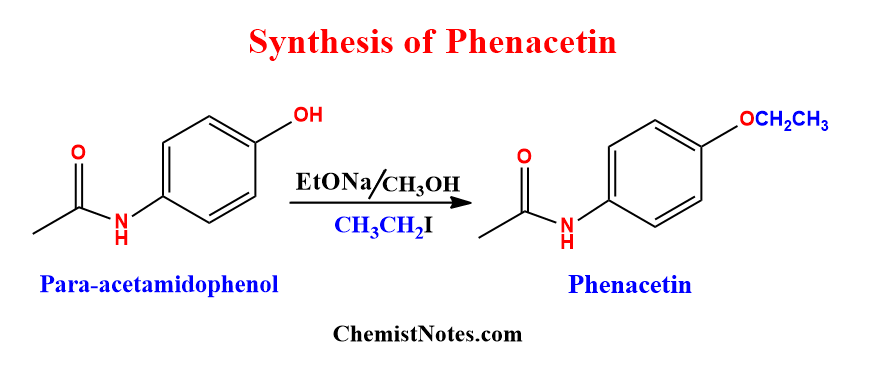
Williamson ether synthesis of phenacetin
Phenacetin is an analgesic and antipyretic drug that is used as a pain reliever and has fewer reducers. This compound can be synthesized via Williamson etherification. When para-acetamidophenol reacts with ethyl iodide in the presence of sodium hydroxide or sodium ethoxide and methanol, it gives phenacetin.
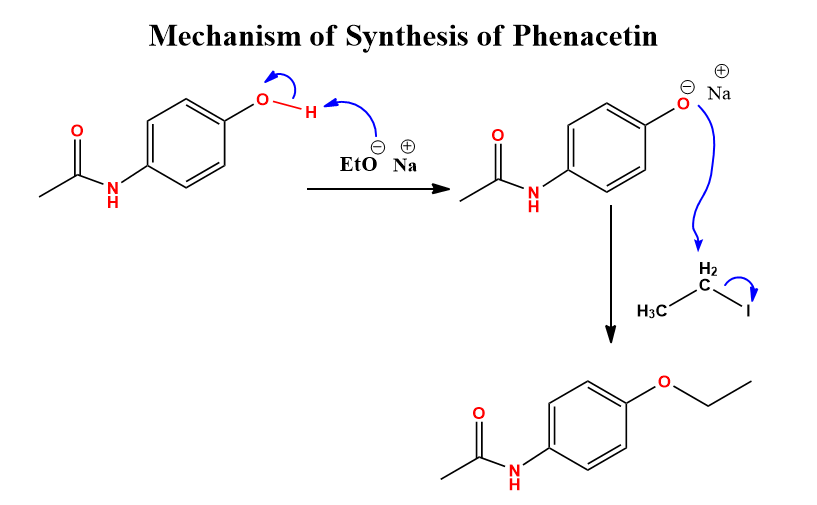
Williamson ether synthesis of phenacetin mechanism
When para-acetamidophenol reacts with sodium hydroxide, it forms sodium para-acetamido phenolate. This reacts with ethyl iodide to form phenacetin. The mechanism is illustrated below:
Williamson ether synthesis of acetophenetidin
Acetophenetidin is also known as Phenacetin. Its synthesis is already given above. Please check it out.
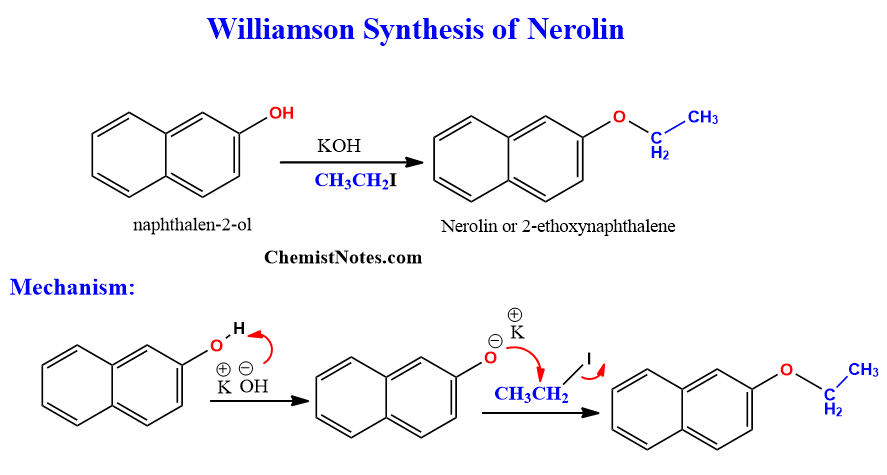
Williamson ether synthesis of Nerolin
Nerolin, also known as 2-ethoxynaphthalene, can be synthesized using β-naphthol and ethyl iodide in the presence of potassium hydroxide.
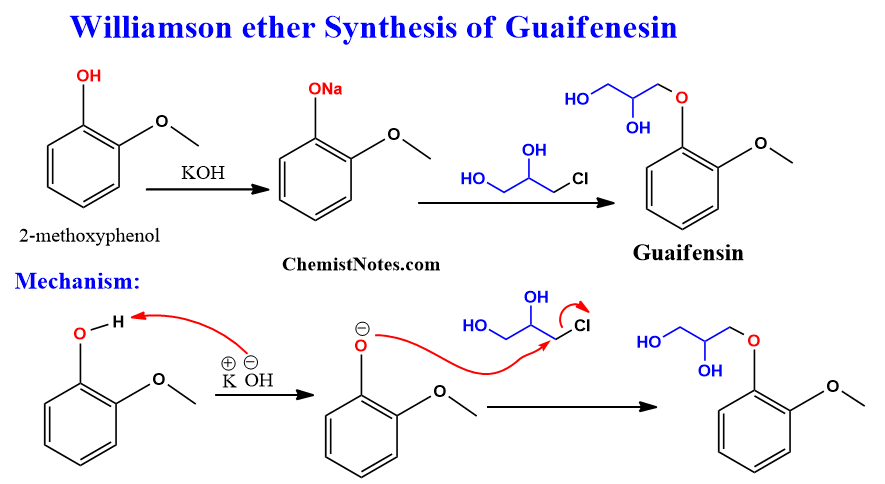
Williamson ether synthesis of guaifenesin
Guaifenesin is a drug that is used to relieve cough and congestion associated with respiratory conditions. This compound can be synthesized by using 2-methoxy phenol and 3-chloropropane-1,2-diol in the presence of a base. The base deprotonates 2-methoxy phenol and the resulting phenolate ion attacks the 3-chloropropane-1,2-diol to form guaifensin.